Peroxynitrite decomposition catalyst reduces vasopressin requirement in ovine MRSA sepsis
- PMID: 31512009
- PMCID: PMC6738358
- DOI: 10.1186/s40635-019-0227-4
Peroxynitrite decomposition catalyst reduces vasopressin requirement in ovine MRSA sepsis
Abstract
Background: Sepsis is one of the most frequent causes of death in the intensive care unit. Host vascular hypo-responsiveness to vasopressors during septic shock is one of the challenging problems. This study tested the hypothesis that adjunct therapy with peroxynitrite decomposition catalyst (WW-85) would reduce arginine vasopressin (AVP) requirements during sepsis resuscitation, using ovine sepsis model.
Methods: Thirteen adult female Merino sheep, previously instrumented with multiple vascular catheters, were subjected to "two-hit" (cotton smoke inhalation and intrapulmonary instillation of live methicillin-resistant Staphylococcus aureus; 3.5 × 1011 colony-forming units) injury. Post injury, animals were awakened and randomly allocated to the following groups: (1) AVP: injured, fluid resuscitated, and titrated with AVP, n = 6 or (2) WW-85 + AVP: injured, fluid resuscitated, treated with WW-85, and titrated with AVP, n = 7. One-hour post injury, a bolus intravenous injection of WW-85 (0.1 mg/kg) was followed by a 23-h continuous infusion (0.02 mg/kg/h). Titration of AVP started at a dose of 0.01 unit/min, when mean arterial pressure (MAP) decreased by 10 mmHg from baseline, despite aggressive fluid resuscitation, and the rate was further adjusted to maintain MAP. After the injury, all animals were placed on a mechanical ventilator and monitored in the conscious state for 24 h.
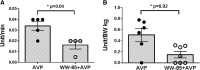
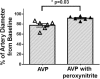
Results: The injury induced severe hypotension refractory to aggressive fluid resuscitation. High doses of AVP were required to partially attenuate the sepsis-induced hypotension. However, the cumulative AVP requirement was significantly reduced by adjunct treatment with WW-85 at 17-24 h after the injury (p < 0.05). Total AVP dose and the highest AVP rate were significantly lower in the WW-85 + AVP group compared to the AVP group (p = 0.02 and 0.04, respectively). Treatment with WW-85 had no adverse effects. In addition, the in vitro effects of AVP on isolated artery diameter changes were abolished with peroxynitrite co-incubation.
Conclusions: The modulation of reactive nitrogen species, such as peroxynitrite, may be considered as a novel adjunct treatment option for septic shock associated with vascular hypo-responsiveness to vasopressors.
Keywords: Arginine vasopressin; Peroxynitrite decomposition catalyst; Refractory shock; Septic shock; Vascular hypo-responsiveness; WW-85.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Modulation of Peroxynitrite Reduces Norepinephrine Requirements in Ovine MRSA Septic Shock.Shock. 2019 Nov;52(5):e92-e99. doi: 10.1097/SHK.0000000000001297. Shock. 2019. PMID: 30499879 Free PMC article.
-
Selective V(1a) agonism attenuates vascular dysfunction and fluid accumulation in ovine severe sepsis.Am J Physiol Heart Circ Physiol. 2012 Nov 15;303(10):H1245-54. doi: 10.1152/ajpheart.00390.2012. Epub 2012 Sep 7. Am J Physiol Heart Circ Physiol. 2012. PMID: 22961865 Free PMC article.
-
The peroxynitrite catalyst WW-85 improves pulmonary function in ovine septic shock.Shock. 2011 Feb;35(2):148-55. doi: 10.1097/SHK.0b013e3181eb4556. Shock. 2011. PMID: 20577150 Free PMC article.
-
[Arginine-vasopressin in septic and vasodilatorial shock].Anasthesiol Intensivmed Notfallmed Schmerzther. 2006 Nov;41(11):716-9. doi: 10.1055/s-2006-958842. Anasthesiol Intensivmed Notfallmed Schmerzther. 2006. PMID: 17151983 Review. German.
-
Role of vasopressin in current anesthetic practice.Korean J Anesthesiol. 2017 Jun;70(3):245-257. doi: 10.4097/kjae.2017.70.3.245. Epub 2017 May 26. Korean J Anesthesiol. 2017. PMID: 28580075 Free PMC article. Review.
Cited by
-
The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies.Int J Mol Sci. 2021 Apr 28;22(9):4642. doi: 10.3390/ijms22094642. Int J Mol Sci. 2021. PMID: 33924958 Free PMC article. Review.
-
Arginine vasopressin receptor 2 activation promotes microvascular permeability in sepsis.Pharmacol Res. 2021 Jan;163:105272. doi: 10.1016/j.phrs.2020.105272. Epub 2020 Nov 4. Pharmacol Res. 2021. PMID: 33160069 Free PMC article.
-
Macrophage-Produced Peroxynitrite Induces Antibiotic Tolerance and Supersedes Intrinsic Mechanisms of Persister Formation.Infect Immun. 2021 Sep 16;89(10):e0028621. doi: 10.1128/IAI.00286-21. Epub 2021 Jun 7. Infect Immun. 2021. PMID: 34097475 Free PMC article.
-
An electrophysiological evaluation method for the ovine facial nerve.Regen Ther. 2021 Apr 16;18:76-81. doi: 10.1016/j.reth.2021.03.008. eCollection 2021 Dec. Regen Ther. 2021. PMID: 33969162 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

