Evaluating Neurodevelopmental Consequences of Perinatal Exposure to Antiretroviral Drugs: Current Challenges and New Approaches
- PMID: 31512167
- PMCID: PMC7064402
- DOI: 10.1007/s11481-019-09880-z
Evaluating Neurodevelopmental Consequences of Perinatal Exposure to Antiretroviral Drugs: Current Challenges and New Approaches
Abstract
As antiretroviral therapy (ART) becomes increasingly affordable and accessible to women of childbearing age across the globe, the number of children who are exposed to Human Immunodeficiency Viruses (HIV) but remain uninfected is on the rise, almost all of whom were also exposed to ART perinatally. Although ART has successfully aided in the decline of mother-to-child-transmission of HIV, the long-term effects of in utero exposure to ART on fetal and postnatal neurodevelopment remain unclear. Evaluating the safety and efficacy of therapeutic drugs for pregnant women is a challenge due to the historic limitations on their inclusion in clinical trials and the dynamic physiological states during pregnancy that can alter the pharmacokinetics of drug metabolism and fetal drug exposure. Thus, much of our data on the potential consequences of ART drugs on the developing nervous system comes from preclinical animal models and clinical observational studies. In this review, we will discuss the current state of knowledge and existing approaches to investigate whether ART affects fetal brain development, and describe novel human stem cell-based strategies that may provide additional information to better predict the impact of specific drugs on the human central nervous system. Graphical Abstract Approaches to evaluate the impact of drugs on the developing brain. Dysregulation of the developing nervous system can lead to long-lasting changes. Integration of data from animal models, clinical observations, and cell culture studies is needed to predict the safety of therapeutic antiretroviral drugs during pregnancy. New approaches include human induced pluripotent stem cell (iPSC)-based 2D and 3D models of neuronal networks and brain regions, as well as single cell profiling in response to drug exposure.
Keywords: Neurodevelopment; antiretroviral drugs; iPSCs; organoids.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Figures

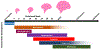
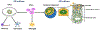
References
-
- Alimenti A, Forbes JC, Oberlander TF, Money DM, Grunau RE, Papsdorf MP, … Burdge DR (2006). A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics, 118(4), e1139–1145. doi:10.1542/peds.2006-0525 - DOI - PubMed
-
- Allavena C, Le Moal G, Michau C, Chiffoleau A, & Raffi F (2006). Neuropsychiatric adverse events after switching from an antiretroviral regimen containing efavirenz without tenofovir to an efavirenz regimen containing tenofovir: a report of nine cases. Antivir Ther, 11(2), 263–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

