Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma
- PMID: 31512258
- PMCID: PMC6899718
- DOI: 10.1002/ajh.25638
Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma
Abstract
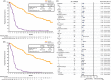
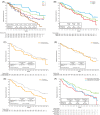
Ibrutinib, a once-daily oral inhibitor of Bruton's tyrosine kinase, is approved in the United States and Europe for treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). The phase 3 RESONATE study showed improved efficacy of single-agent ibrutinib over ofatumumab in patients with relapsed/refractory CLL/SLL, including those with high-risk features. Here we report the final analysis from RESONATE with median follow-up on study of 65.3 months (range, 0.3-71.6) in the ibrutinib arm. Median progression-free survival (PFS) remained significantly longer for patients randomized to ibrutinib vs ofatumumab (44.1 vs 8.1 months; hazard ratio [HR]: 0.148; 95% confidence interval [CI]: 0.113-0.196; P˂.001). The PFS benefit with ibrutinib vs ofatumumab was preserved in the genomic high-risk population with del(17p), TP53 mutation, del(11q), and/or unmutated IGHV status (median PFS 44.1 vs 8.0 months; HR: 0.110; 95% CI: 0.080-0.152), which represented 82% of patients. Overall response rate with ibrutinib was 91% (complete response/complete response with incomplete bone marrow recovery, 11%). Overall survival, censored for crossover, was better with ibrutinib than ofatumumab (HR: 0.639; 95% CI: 0.418-0.975). With up to 71 months (median 41 months) of ibrutinib therapy, the safety profile remained consistent with prior reports; cumulatively, all-grade (grade ≥3) hypertension and atrial fibrillation occurred in 21% (9%) and 12% (6%) of patients, respectively. Only 16% discontinued ibrutinib because of adverse events (AEs). These long-term results confirm the robust efficacy of ibrutinib in relapsed/refractory CLL/SLL irrespective of high-risk clinical or genomic features, with no unexpected AEs. This trial is registered at www.clinicaltrials.gov (NCT01578707).
© 2019 The Authors. American Journal of Hematology published by Wiley Periodicals, Inc.
Conflict of interest statement
T.M. has received honoraria from Janssen, AbbVie, Gilead, Alexion, Novartis, and Roche; and has been in a consulting role for MorphoSys and Sunesis. J.R.B. has received honoraria from Janssen and Teva; has been in a consulting role for AbbVie, Acerta, Astellas, BeiGene, Genentech/Roche, Gilead, Juno/Celgene, Kite, Loxo, Novartis, Pfizer, Pharmacyclics LLC, an AbbVie Company, Redx, Sun, Sunesis, TG Therapeutics, and Verastem; has received research funding from Gilead, Loxo, Sun, and Verastem; and has served on data safety monitoring committees for MorphoSys and Invectys. S.O. has been in a consulting role for Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences, Vaniam Group, AbbVie, Alexion, Verastem, and Eisai; has received research funding from Kite, Regeneron, and Acerta; and has been in a consulting role and received research funding from Gilead, Pharmacyclics LLC, an AbbVie Company, TG Therapeutics, Pfizer, and Sunesis. J.C.Ba. has received honoraria from Janssen; has been in a consulting role for Genentech, Gilead, Bayer, Pharmacyclics LLC, an AbbVie Company, AbbVie, AstraZeneca, and Sandoz; and has received research funding from Pharmacyclics LLC, an AbbVie Company, Oncternal Therapeutics, and AbbVie. P.M.B. has been in a consulting role for Pharmacyclics LLC, an AbbVie Company, and AbbVie, and has received research funding from Pharmacyclics LLC, an AbbVie Company. N.M.R. has received honoraria from and has been in a consulting role for Bristol‐Myers Squibb (BMS), Genentech, AbbVie, Gilead, AstraZeneca, and Celgene; has received research funding from BMS and Merck; and has been a paid speaker for Gilead and Celgene. S.C. has received honoraria from Pharmacyclics LLC, an AbbVie Company, and Janssen; has been in a consulting role for AbbVie, Celgene, Genentech, Pharmacyclics LLC, an AbbVie Company, Janssen, Novartis, Astellas, and AstraZeneca; has received research funding from AbbVie, Acerta, Celgene, Gilead, Janssen, Pharmacyclics LLC, an AbbVie Company, and Takeda; has provided expert testimony for Genentech; has received travel, accommodations and other expenses from AbbVie, BeiGene, Celgene, Genentech, Janssen, and Pharmacyclics LLC, an AbbVie Company; and has other relationships with BeiGene. C.S.T. has received honoraria from Janssen and Pharmacyclics LLC, an AbbVie Company, and research funding from Janssen. S.P.M. has received honoraria from and has been in a consulting role for Roche, AbbVie, Janssen, Gilead, and GlaxoSmithKline; has received research funding from Roche, AbbVie, and Janssen; and has been a paid speaker for Roche, AbbVie, Janssen, and Gilead. U.J. has received honoraria and research funding from Janssen, and has been in a consulting role for Janssen. T.J.K. has been in a consulting role for AbbVie, Genentech‐Roche, Gilead, Pharmacyclics LLC, an AbbVie Company, and Celgene and has received research funding from AbbVie, Genentech‐Roche, Pharmacyclics LLC, an AbbVie Company, and Oncternal. C.M. has been in a consulting role for Pharmacyclics LLC, an AbbVie Company, Janssen, and AbbVie. M.M. has received honoraria from and has been in a consulting role for AbbVie, Janssen, Gilead, Roche, and AstraZeneca; has been a paid speaker for AbbVie, Janssen, and Gilead; has received research funding from Roche; and has received travel, accommodations, and expenses reimbursement from AbbVie, Janssen, and AstraZeneca. J.A.B. has received honoraria from Janssen; has been in a consulting role for Janssen; has received research funding from Gilead, TG Therapeutics, Pharmacyclics LLC, an AbbVie Company, and BeiGene; has been a paid speaker for Gilead, TG Therapeutics, Pharmacyclics LLC, an AbbVie Company, Novartis, and Janssen; and has received travel, accommodations, and expenses reimbursement from Gilead, TG Therapeutics, Pharmacyclics LLC, an AbbVie Company, Novartis, and Janssen. J.C.By. has been in consulting role for Janssen; has reported research funding from Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, and BeiGene; and has been a paid speaker for Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, Novartis, and Janssen; and received travel, accommodations, and expenses reimbursement from Pharmacyclics LLC, an AbbVie Company, Janssen, Novartis, Gilead, and TG Therapeutics. P.H. has received honoraria from, has been in a consulting role for and has received research funding from Janssen, Pharmacyclics LLC, an AbbVie Company, and AbbVie; and has received travel, accommodations, and expenses reimbursement from Janssen and AbbVie. S.D. is an employee of Pharmacyclics LLC, an AbbVie Company, and owns stock in AbbVie, Celgene, Gilead, GlaxoSmithKline, and Exelixis. A.S. is an employee of Pharmacyclics LLC, an AbbVie Company, and owns stock in AbbVie. J.P.D. is an employee of Pharmacyclics LLC, an AbbVie Company, and owns stock in AbbVie and was formerly employed by and owned stock in CTI BioPharma. J.W. has been in a consulting role for Pharmacyclics LLC, an AbbVie Company, and Janssen; and has received research funding from Pharmacyclics LLC, an AbbVie Company, Janssen, AbbVie, Morphosys, Karyopharm, and Loxo.
Figures

Comment in
-
A final note about ibrutinib in relapsed or refractory CLL: Conclusive results from RESONATE sound definitely good!Am J Hematol. 2019 Dec;94(12):1303-1305. doi: 10.1002/ajh.25662. Epub 2019 Nov 7. Am J Hematol. 2019. PMID: 31621103 No abstract available.
References
-
- Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017;92(9):946‐965. - PubMed
-
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910‐1916. - PubMed
-
- Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848‐1854. - PubMed
-
- Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840‐1847. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
