A Phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas
- PMID: 31512497
- PMCID: PMC6854438
- DOI: 10.2217/fon-2019-0209
A Phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas
Abstract
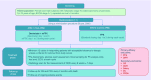
Devimistat (CPI-613®) is a novel lipoate analog that inhibits the tricarboxcylic acid cycle at two key carbon entry points. Through its inhibition of pyruvate dehydrogenase and a-ketoglutarate dehydrogenase complexes, devimistat inhibits the entry of glucose and glutamine derived carbons, respectively. Pancreatic cancer is dependent on mitochondrial function for enhanced survival and aggressiveness. In a Phase I study of modified FOLFIRINOX, in combination with devimistat for metastatic pancreatic cancer patients, there was a 61% objective response rate including a 17% complete response rate. This report outlines the rationale and design of the AVENGER 500 study, a Phase III clinical trial of devimistat in combination with modified FOLFIRINOX compared with FOLFIRINOX alone for patients with previously untreated metastatic adenocarcinoma of the pancreas. Clinical trial registration: NCT03504423.
Keywords: CPI-613; FOLFIRINOX; Phase III; metastatic; pancreatic cancer.
Conflict of interest statement
AT Alistar has been a consultant for Rafael Pharmaceuticals, S Luther and TS Pardee are employees of Rafael Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 388(10039), 73–85 (2016). - PubMed
-
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N. Engl. J. Med. 371(11), 1039–1049 (2014). - PubMed
-
• An excellent review on pancreatic cancer outlining the clinical and biological features of the disease.
-
- Conroy T, Desseigne F, Ychou M. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364(19), 1817–1825 (2011). - PubMed
-
• Reports the Phase III results of FOLOFIRINOX in metastatic pancreatic cancer patients.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical