Research Status of Mesenchymal Stem Cells in Liver Transplantation
- PMID: 31512503
- PMCID: PMC6923564
- DOI: 10.1177/0963689719874786
Research Status of Mesenchymal Stem Cells in Liver Transplantation
Abstract
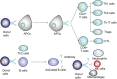
Liver transplantation has been deemed the best choice for end-stage liver disease patients but immune rejection after surgery is still a serious problem. Patients have to take immunosuppressive drugs for a long time after liver transplantation, and this often leads to many side effects. Mesenchymal stem cells (MSCs) gradually became of interest to researchers because of their powerful immunomodulatory effects. In the past, a large number of in vitro and in vivo studies have demonstrated the great potential of MSCs for participation in posttransplant immunomodulation. In addition, MSCs also have properties that may potentially benefit patients undergoing liver transplantation. This article aims to provide an overview of the current understanding of the immunomodulation achieved by the application of MSCs in liver transplantation, to discuss the problems that may be encountered when using MSCs in clinical practice, and to describe some of the underlying capabilities of MSCs in liver transplantation. Cell-cell contact, soluble molecules, and exosomes have been suggested to be critical approaches to MSCs' immunoregulation in vitro; however, the exact mechanism, especially in vivo, is still unclear. In recent years, the clinical safety of MSCs has been proven by a series of clinical trials. The obstacles to the clinical application of MSCs are decreasing, but large sample clinical trials involving MSCs are still needed to further study their clinical effects.
Keywords: clinical trial; immunosuppression; liver transplantation; mesenchymal stromal cells; tolerance.
Conflict of interest statement
Figures
Similar articles
-
Mesenchymal stem cell therapy for liver transplantation: clinical progress and immunomodulatory properties.Stem Cell Res Ther. 2024 Sep 27;15(1):320. doi: 10.1186/s13287-024-03943-6. Stem Cell Res Ther. 2024. PMID: 39334441 Free PMC article. Review.
-
Steroid-Mediated Decrease in Blood Mesenchymal Stem Cells in Liver Transplant could Impact Long-Term Recovery.Stem Cell Rev Rep. 2017 Oct;13(5):644-658. doi: 10.1007/s12015-017-9751-3. Stem Cell Rev Rep. 2017. PMID: 28733800
-
Immunomodulation effect of mesenchymal stem cells in islet transplantation.Biomed Pharmacother. 2021 Oct;142:112042. doi: 10.1016/j.biopha.2021.112042. Epub 2021 Aug 14. Biomed Pharmacother. 2021. PMID: 34403963 Review.
-
Immunomodulatory Role of Mesenchymal Stem Cells in Liver Transplantation: Status and Prospects.Dig Dis. 2024;42(1):41-52. doi: 10.1159/000534003. Epub 2023 Sep 20. Dig Dis. 2024. PMID: 37729883 Review.
-
Bone marrow-derived mesenchymal stem cells as immunosuppressants in liver transplantation: a review of current data.Transfus Med Rev. 2012 Apr;26(2):129-41. doi: 10.1016/j.tmrv.2011.09.002. Epub 2011 Oct 19. Transfus Med Rev. 2012. PMID: 22015073 Review.
Cited by
-
Hypoxia-induced shift in the phenotype of proteasome from 26S toward immunoproteasome triggers loss of immunoprivilege of mesenchymal stem cells.Cell Death Dis. 2020 Jun 4;11(6):419. doi: 10.1038/s41419-020-2634-6. Cell Death Dis. 2020. PMID: 32499535 Free PMC article.
-
Perspective of placenta derived mesenchymal stem cells in acute liver failure.Cell Biosci. 2020 May 24;10:71. doi: 10.1186/s13578-020-00433-z. eCollection 2020. Cell Biosci. 2020. PMID: 32483484 Free PMC article. Review.
-
Regulatory Immune Cell-derived Exosomes: Modes of Action and Therapeutic Potential in Transplantation.Transplantation. 2025 Jul 1;109(7):1124-1137. doi: 10.1097/TP.0000000000005309. Epub 2025 Jan 27. Transplantation. 2025. PMID: 39865513 Review.
-
Therapeutic Mesenchymal Stem/Stromal Cells: Value, Challenges and Optimization.Front Cell Dev Biol. 2022 Jan 14;9:716853. doi: 10.3389/fcell.2021.716853. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35096805 Free PMC article. Review.
-
Mesenchymal stem cells transfected with sFgl2 inhibit the acute rejection of heart transplantation in mice by regulating macrophage activation.Stem Cell Res Ther. 2020 Jun 17;11(1):241. doi: 10.1186/s13287-020-01752-1. Stem Cell Res Ther. 2020. PMID: 32552823 Free PMC article.
References
-
- Chascsa DM, Vargas HE. The gastroenterologist’s guide to management of the post-liver transplant patient. Am J Gastroenterol. 2018;113(6):819–28. - PubMed
-
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol. 2019;70(4):745–58. - PubMed
-
- Bottomley MJ, Harden PN. Update on the long-term complications of renal transplantation. Br Med Bull. 2013;106:117–34. - PubMed
-
- Casiraghi F, Perico N, Cortinovis M, Remuzzi G. Mesenchymal stromal cells in renal transplantation: opportunities and challenges. Nat Rev Nephrol. 2016;12(4):241–53. - PubMed
-
- Hinden L, Shainer R, Almogi-Hazan O, Or R. Ex Vivo induced regulatory human/murine mesenchymal stem cells as immune modulators. Stem Cells. 2015;33(7):2256–67. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

