Interventions for maintenance of surgically induced remission in Crohn's disease: a network meta-analysis
- PMID: 31513295
- PMCID: PMC6741529
- DOI: 10.1002/14651858.CD013210.pub2
Interventions for maintenance of surgically induced remission in Crohn's disease: a network meta-analysis
Abstract
Background: Crohn's disease (CD) is a chronic disease of the gut. About 75% of people with CD undergo surgery at least once in their lifetime to induce remission. However, as there is no known cure for the disease, patients usually experience a recurrence even after surgery. Different interventions are routinely used in maintaining postsurgical remission. There is currently no consensus on which treatment is the most effective.
Objectives: To assess the effects and harms of interventions for the maintenance of surgically induced remission in Crohn's disease and rank the treatments in order of effectiveness.
Search methods: We searched the Cochrane IBD Group Specialized Register, CENTRAL, MEDLINE, and Embase from inception to 15 January 2019. We also searched reference lists of relevant articles, abstracts from major gastroenterology meetings, ClinicalTrials.gov, and the WHO ICTRP. There was no restriction on language, date, or publication status.
Selection criteria: We considered for inclusion randomised controlled trials (RCTs) that compared different interventions used for maintaining surgically induced remission in people with CD who were in postsurgical remission. Participants had to have received maintenance treatment for at least three months. We excluded studies assessing enteral diet, diet manipulation, herbal medicine, and nutritional supplementation.
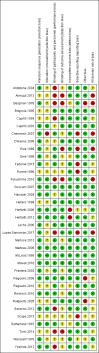
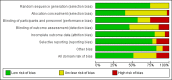
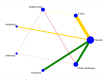
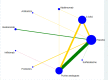
Data collection and analysis: Two review authors independently selected relevant studies, extracted data, and assessed the risk of bias. Any disagreements were resolved by discussion or by arbitration of a third review author when necessary. We conducted a network meta-analysis (NMA) using a Bayesian approach through Markov Chain Monte Carlo (MCMC) simulation. For the pairwise comparisons carried out in Review Manager 5, we calculated risk ratios (RR) with their corresponding 95% confidence intervals (95% CI). For the NMA, we presented hazard ratios (HR) with corresponding 95% credible intervals (95% CrI) and reported ranking probabilities for each intervention. For the NMA, we focused on three main outcomes: clinical relapse, endoscopic relapse, and withdrawals due to adverse events. Data were insufficient to assess time to relapse and histologic relapse. Adverse events and serious adverse events were not sufficiently or objectively reported to permit an NMA. We used CINeMA (Confidence in Network Meta-Analysis) methods to evaluate our confidence in the findings within networks, and GRADE for entire networks.
Main results: We included 35 RCTs (3249 participants) in the review. The average age of study participants ranged between 33.6 and 38.8 years. Risk of bias was high in 18 studies, low in four studies, and unclear in 13 studies. Of the 35 included RCTs, 26 studies (2581 participants; 9 interventions) were considered eligible for inclusion in the NMA. The interventions studied included 5-aminosalicylic acid (5-ASA), adalimumab, antibiotics, budesonide, infliximab, probiotics, purine analogues, sulfasalazine, and a combination of sulfasalazine and prednisolone. This resulted in 30 direct contrasts, which informed 102 mixed-treatment contrasts.The evidence for the clinical relapse network (21 studies; 2245 participants) and endoscopic relapse (12 studies; 1128 participants) were of low certainty while the evidence for withdrawal due to adverse events (15 studies; 1498 participants) was of very low certainty. This assessment was due to high risk of bias in most of the studies, inconsistency, and imprecision across networks. We mainly judged individual contrasts as of low or very low certainty, except 5-ASA versus placebo, the evidence for which was judged as of moderate certainty.We ranked the treatments based on effectiveness and the certainty of the evidence. For clinical relapse, the five most highly ranked treatments were adalimumab, infliximab, budesonide, 5-ASA, and purine analogues. We found some evidence that adalimumab (HR 0.11, 95% Crl 0.02 to 0.33; low-certainty evidence) and 5-ASA may reduce the probability of clinical relapse compared to placebo (HR 0.69, 95% Crl 0.53 to 0.87; moderate-certainty evidence). However, budesonide may not be effective in preventing clinical relapse (HR 0.66, 95% CrI 0.27 to 1.34; low-certainty evidence). We are less confident about the effectiveness of infliximab (HR 0.36, 95% CrI 0.02 to 1.74; very low-certainty evidence) and purine analogues (HR 0.75, 95% CrI 0.55 to 1.00; low-certainty evidence). It was unclear whether the other interventions reduced the probability of a clinical relapse, as the certainty of the evidence was very low.Due to high risk of bias and limited data across the network, we are uncertain about the effectiveness of interventions for preventing endoscopic relapse. Whilst there might be some evidence of prevention of endoscopic relapse with adalimumab (HR 0.10, 95% CrI 0.01 to 0.32; low-certainty evidence), no other intervention studied appeared to be effective.Due to high risk of bias and limited data across the network, we are uncertain about the effectiveness of interventions for preventing withdrawal due to adverse events. Withdrawal due to adverse events appeared to be least likely with sulfasalazine (HR 1.96, 95% Crl 0.00 to 8.90; very low-certainty evidence) and most likely with antibiotics (HR 53.92, 95% Crl 0.43 to 259.80; very low-certainty evidence). When considering the network as a whole, two adverse events leading to study withdrawal (i.e. pancreatitis and leukopenia) occurred in more than 1% of participants treated with an intervention. Pancreatitis occurred in 2.8% (11/399) of purine analogue participants compared to 0.17% (2/1210) of all other groups studied. Leukopenia occurred in 2.5% (10/399) of purine analogue participants compared to 0.08% (1/1210) of all other groups studied.
Authors' conclusions: Due to low-certainty evidence in the networks, we are unable to draw conclusions on which treatment is most effective for preventing clinical relapse and endoscopic relapse. Evidence on the safety of the interventions was inconclusive, however cases of pancreatitis and leukopenia from purine analogues were evident in the studies. Larger trials are needed to further understand the effect of the interventions on endoscopic relapse.
Conflict of interest statement
Zipporah Iheozor‐Ejiofor: None known.
Morris Gordon has received travel fees from Abbott, Nutricia, BioGaia, Ferring, Allergan, and Tillots to attend international scientific and training meetings such as DDW, Advances in IBD, ESPGHAN, BSPGHAN, and Cochrane‐focused international events. None of these companies has had any involvement in any works completed by Morris Gordon, and he has had no payments for any other activities.
Andrew Clegg: None known.
Suzanne C Freeman: None known
Teuta Gjuladin‐Hellon: None known.
John K MacDonald: None known.
Anthony K Akobeng: None known.
Figures



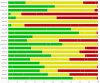
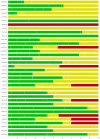
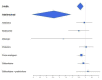
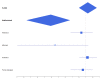

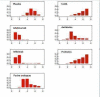
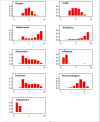
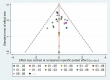






































































Update of
- doi: 10.1002/14651858.CD013210
References
References to studies included in this review
Ardizzone 2004 {published data only}
-
- Ardizzone S, MacOni G, Sampietro GM, Russo A, Radice E, Colombo E, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology 2004;127(3):730‐40. - PubMed
Armuzzi 2013 {published data only}
-
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Prevention of postoperative recurrence with azathioprine or anti‐TNF alpha in patients with Crohn's disease: an open‐label pilot study. Gastroenterology 2012;142(5):S780. - PubMed
-
- Armuzzi A, Felice C, Papa A, Marzo M, Pugliese D, Andrisani G, et al. Prevention of postoperative recurrence with azathioprine or infliximab in patients with Crohn's disease: an open‐label pilot study. Journal of Crohn's and Colitis 2013;7(12):623‐9. - PubMed
Bergman 1976 {published data only}
-
- Bergman L, Krause U. Postoperative treatment with corticosteroids and salazosulphapyridine (Salazopyrin) after radical resection for Crohn's disease. Scandinavian Journal of Gastroenterology 1976;11(7):651‐6. - PubMed
Brignola 1995 {published data only}
-
- Brignola C, Cottone M, Pera A, Ardizzone S, Scribano ML, Franchis R, et al. Mesalamine in the prevention of endoscopic recurrence after intestinal resection for Crohn's disease. Gastroenterology 1995;108(2):345‐9. - PubMed
Caprilli 1994 {published data only}
-
- Caprilli R, Andreoli A, Capurso L, Corrao G, D'Albasio G, Gioieni A. Oral mesalazine (5‐aminosalicylic acid; Asacol) for the prevention of post‐operative recurrence of Crohn's disease. Alimentary Pharmacology & Therapeutics 1994;8(1):35‐43. - PubMed
Caprilli 2003 {published data only}
-
- Caprilli R, Cottone M, Tonelli F, Sturniolo G, Castiglione F, Annese V, et al. Two mesalazine regimens in the prevention of the post‐operative recurrence of Crohn's disease: a pragmatic, double‐blind, randomized controlled trial. Alimentary Pharmacology & Therapeutics 2003;17(4):517‐23. - PubMed
Chermesh 2007 {published data only}
-
- Chermesh I, Tamir A, Reshef R, Chowers Y, Suissa A, Katz D, et al. Failure of Synbiotic 2000 to prevent postoperative recurrence of Crohn's disease. Digestive Diseases and Sciences 2007;52(2):385‐9. - PubMed
D'Haens 2008 {published data only}
-
- D'Haens GR, Vermeire S, Assche G, Noman M, Aerden I, Olmen G, et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn's Disease: a controlled randomized trial. Gastroenterology 2008;135(4):1123‐9. - PubMed
Ewe 1989 {published data only}
-
- Ewe K, Herfarth C, Malchow H, Jesdinsky HJ. Postoperative recurrence of Crohn's disease in relation to radicality of operation and sulfasalazine prophylaxis: a multicenter trial. Digestion 1989;42(4):224‐32. - PubMed
Ewe 1999 {published data only}
-
- Ewe K, Böttger T, Buhr HJ, Ecker KW, Otto HF. Low‐dose budesonide treatment for prevention of postoperative recurrence of Crohn's disease: a multicentre randomized placebo‐controlled trial. European Journal of Gastroenterology & Hepatology 1999;11(3):277‐82. - PubMed
Fedorak 2015 {published data only}
-
- Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, et al. The probiotic VSL#3 has anti‐inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Gastroenterology and Hepatology 2015;13:928‐35. - PubMed
-
- NCT00175292. A randomized controlled trial of VSL#3 for the prevention of endoscopic recurrence following surgery for Crohn's. clinicaltrials.gov/ct2/show/NCT00175292 (first received 15 September 2005).
Florent 1996 {published data only}
-
- Florent C, Cortot A, Quandale P, Sahmound T, Modigliani R, Sarfaty E, et al. Placebo‐controlled clinical trial of mesalazine in the prevention of early endoscopic recurrences after resection for Crohn's disease. European Journal of Gastroenterology & Hepatology 1996;8(3):229‐33. - PubMed
Fukushima 2018 {published data only}
-
- Fukushima K, Sugita A, Futami K, Takahashi KI, Motoya S, Kimura H, et al. Postoperative therapy with infliximab for Crohn's disease: a 2‐year prospective randomized multicenter study in Japan. Surgery Today 2018;48(6):584‐90. - PubMed
-
- JPRN‐UMIN000002604. Combined therapy of infliximab with surgery in maintenance of remission in Crohn's disease. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000003157 (first received 1 November 2009).
Gossum 2007 {published data only}
-
- Gossum A, Dewit O, Louis E, Hertogh G, Baert F, Fontaine F, et al. Multicenter randomized‐controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn's disease after lleo‐caecal resection. Inflammatory Bowel Diseases 2007;13(2):135‐42. - PubMed
Hanauer 2004 {published data only}
-
- Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, et al. Can we prevent the inevitable? The use of 6‐mercaptopurine or mesalamine to prevent postoperative recurrence in patients with Crohn disease. Evidence‐Based Gastroenterology 2005;6(1):17‐9.
-
- Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, et al. Postoperative maintenance of Crohn's disease remission with 6‐mercaptopurine, mesalamine, or placebo: a 2‐year trial. Gastroenterology 2004;127(3):723‐9. - PubMed
Hellers 1999 {published data only}
-
- Hellers G, Cortot A, Jewell D, Leijonmarck CE, Lofberg R, Malchow H, et al. Oral budesonide for prevention of postsurgical recurrence in Crohn's disease. Gastroenterology 1999;116(2):294‐300. - PubMed
Herfarth 2006 {published data only}
-
- Herfarth H, Obermeier F, Tjaden C, Lukas M, Serclova Z, Dignass AU, et al. Double‐blind, double dummy, randomized, multicentre, comparative study on the efficacy and safety of azathioprine (AZA) versus mesalazine (5‐ASA) for prevention of postoperative endoscopic recurrence in Crohn’s disease. Gastroenterology 2006; Vol. 130, issue 4 Suppl 2:A480‐1.
-
- Muller R, Herfarth H. More information on study in 2006 Gut [personal communication]. Email to: M Gordon 2 May 2012.
Herfarth 2013 {published data only}
-
- NCT00609973. Ciprofloxacin for the prevention of postoperative endoscopic recurrence in Crohn's disease. clinicaltrials.gov/ct2/show/NCT00609973 (first received 7 February 2008).
Lochs 2000 {published data only}
-
- Lochs H, Mayer M, Fleig WE, Mortensen PB, Bauer P, Genser D, et al. Prophylaxis of postoperative relapse in Crohn's disease with mesalamine: European Cooperative Crohn's Disease Study VI. Gastroenterology 2000;118(2):264‐73. - PubMed
Lopez Sanroman 2017 {published data only}
-
- Lopez‐Sanroman A, Vera‐Mendoza I, Domenech E, Taxonera C, Vega V, Marin‐Jimenez I, et al. APPRECIA: adalimumab vs azathioprine in the prevention of Crohn's disease recurrence after surgical resection. A GETECCU multicenter randomized trial. United European Gastroenterology Journal 2015;1:A3.
-
- Lopez‐Sanroman A, Vera‐Mendoza I, Domenech E, Taxonera C, Vega V, Marin‐Jimenez I, et al. Adalimumab vs azathioprine in the prevention of postoperative Crohn’s disease recurrence. A GETECCU randomised trial. Journal of Crohn's and Colitis 2017;11(11):1293‐301. - PubMed
-
- NCT01564823. Adalimumab on preventing post‐chirurgic recurrence on Crohn's disease. clinicaltrials.gov/ct2/show/NCT01564823 (first received 28 March 2012).
-
- Taxonera C, Lopez‐Sanroman A, Vera I, Nos P. Health‐related quality of life improves during one year of postoperative prophylactic drug therapy after ileocecal intestinal resection in Crohn's disease patients: results of the APPRECIA randomized trial. Gastroenterology 2016;1:S442‐3. - PubMed
Mañosa 2013 {published data only}
-
- Manosa M, Cabre E, Bernal I, Esteve M, Garcia‐Planella E, Ricart Gomez E, et al. Azathioprine versus azathioprine plus metronidazole for the prevention of postoperative endoscopic recurrence of Crohn's disease: a randomized, placebo‐controlled trial. Journal of Crohn's and Colitis 2012;1:S93. - PubMed
-
- Mañosa M, Cabré E, Bernal I, Esteve M, Garcia‐Planella E, Ricart E, et al. Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn's disease: a randomized, double‐blind, placebo‐controlled trial. Inflammatory Bowel Diseases 2013;19(9):1889‐95. - PubMed
Marteau 2006 {published data only}
McLeod 1995 {published data only}
-
- McLeod RS, Wolff BG, Steinhart AH, Carryer PW, O'Rourke K, Andrews DF, et al. Prophylactic mesalamine treatment decreases postoperative recurrence of Crohn's disease. Gastroenterology 1995;109(2):404‐13. - PubMed
Mowat 2016 {published data only}
-
- Arnott I, Mowat C, Ennis H, Keerie C, Lewis S, Cahill A, et al. The TOPPIC trial: a randomised, double‐blind parallel group trial of mercaptopurine vs placebo to prevent recurrence of Crohn's disease following surgical resection in 240 patients. Gut 2016;65:A43‐4.
-
- ISRCTN89489788. Randomised controlled trial of 6‐Mercaptopurine (6MP) versus placebo to prevent recurrence of Crohn's disease following surgical resection. isrctn.com/ISRCTN89489788 (first received 15 June 2007).
-
- Mowat C, Arnott I, Cahill A, Smith M, Ahmad T, Subramanian S, et al. Mercaptopurine versus placebo to prevent recurrence of Crohn's disease after surgical resection (TOPPIC): a multicentre, double‐blind, randomised controlled trial. Lancet Gastroenterology and Hepatology 2016;1(4):273‐82. - PMC - PubMed
Prantera 2002 {published data only}
Regueiro 2009 {published data only}
-
- NCT00688636. Infliximab for the prevention of recurrent Crohn's disease after surgery. clinicaltrials.gov/ct2/show/NCT00688636 (first received 3 June 2008).
-
- Regueiro M, El‐Hachem S, Kip KE, Schraut W, Baidoo L, Watson A, et al. Postoperative infliximab is not associated with an increase in adverse events in Crohn's disease. Digestive Diseases and Sciences 2011;56(12):3610‐5. - PubMed
-
- Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, et al. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology 2009;136(2):441‐50. - PubMed
Regueiro 2016 {published data only}
-
- EUCTR2010‐018431‐18‐DE. A multicenter trial comparing REMICADE (infliximab) and placebo in the prevention of recurrence in Crohn's disease patients undergoing surgical resection who are at an increased risk of recurrence. clinicaltrialsregister.eu/ctr‐search/trial/2010‐018431‐18/GB (first received 6 July 2010).
-
- Regueiro M, Feagan BG, Zou B, Johanns J, Blank MA, Chevrier M, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn's disease after ileocolonic resection. Gastroenterology 2016;150(7):1568‐78. - PubMed
Reinisch 2010 {published data only}
-
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar‐Meir S, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomised, double‐blind, double‐dummy, multicentre trial. Gut 2010;59(6):752‐9. - PubMed
-
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Greinwald R, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in Crohn's disease patients with endoscopic recurrence: follow‐up data of a randomised, double‐blind, double‐dummy, 1‐year, multicentre trial. Journal of Crohn's and Colitis 2013;7(1):S254.
Rutgeerts 2005 {published data only}
-
- Rutgeerts P, Assche G, Vermeire S, D'Haens G, Baert F, Noman M, et al. Ornidazole for prophylaxis of postoperative Crohn's disease recurrence: a randomized, double‐blind, placebo‐controlled trial. Gastroenterology 2005;128(4):856‐61. - PubMed
Savarino 2013 {published data only}
-
- Bodini G, Pellegatta G, Giannini EG, Savarino V, Savarino EV. Adalimumab therapy rather than azathioprine and mesalamine is able to halt Crohn's disease progression after resective surgery and a post‐hoc analysis of a prospective randomized study. Gastroenterology 2017;152(5 Suppl 1):S774.
-
- Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. American Journal of Gastroenterology 2013;108:1731‐42. - PubMed
Scapa 2015 {published data only}
-
- Scapa E, Maharshak N, Kariv Y, Ben‐Horin S, White ID, Santo E, et al. Early initiation of adalimumab significantly diminishes post‐operative Crohn's disease recurrence, and is superior to immunomodulator therapy. Preliminary results from the POPART trial. Gastroenterology 2015;148(4 Suppl 1):S240‐1.
Sutherland 1997 {published data only}
-
- Sutherland LR, Martin F, Bailey RJ, Fedorak RN, Poleski M, Dallaire C, et al. A randomized, placebo‐controlled, double‐blind trial of mesalamine in the maintenance of remission of Crohn's disease. The Canadian Mesalamine for Remission of Crohn's Disease Study Group. Gastroenterology 1997;112(4):1069‐77. - PubMed
Tursi 2014 {published data only}
-
- Tursi A, Elisei W, Picchio M, Zampaletta C, Pelecca G, Faggiani R, et al. Comparison of the effectiveness of infliximab and adalimumab in preventing postoperative recurrence in patients with Crohn's disease: an open‐label, pilot study. Techniques in Coloproctology 2014;18(11):1041‐6. - PubMed
Wenckert 1978 {published data only}
-
- Wenckert A, Kristensen M, Eklund AE, Barany F, Jarnum S, Worning H, et al. The long‐term 20 prophylactic effect of salazosulphapyridine (Salazopyrin) in primarily resected patients with 21 Crohn's disease. A controlled double‐blind trial. Scandinavian Journal of Gastroenterology 1978;13(2):161‐7. - PubMed
Yoshida 2012 {published data only}
-
- Yoshida K, Fukunaga K, Ikeuchi H, Kamikozuru K, Hida N, Ohda Y, et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn's disease following ileocolic or ileal resection: a 3‐year prospective randomized open trial. Inflammatory Bowel Diseases 2012;18(9):1617‐23. - PubMed
References to studies excluded from this review
Angelberger 2013 {published data only}
-
- Angelberger S, Schaeffeler E, Teml A, Petritsch W, Shonova O, Lukas M, et al. Mucosal improvement in patients with moderate to severe postoperative endoscopic recurrence of Crohn's disease and azathioprine metabolite levels. Inflammatory Bowel Diseases 2013;19(3):590‐8. - PubMed
Armuzzi 2015 {published data only}
-
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Infliximab is more effective than azathioprine in the long‐term prevention of postoperative recurrence of Crohn's disease. Gastroenterology 2015;148(4):S856.
Balzola 2010 {published data only}
-
- Balzola F, Bernstein C, Assche G. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn's disease: a prospective pilot study: Commentary. Inflammatory Bowel Disease Monitor 2010;10(3):102‐3. - PubMed
Bodini 2014a {published data only}
-
- Bodini G, Cassan C, Savarino V, Savarino E. Letter: Biological therapies are effective for prevention of post‐operative Crohn's disease recurrence. Alimentary Pharmacology and Therapeutics 2014;40(3):322. - PubMed
Bodini 2014b {published data only}
-
- Bodini G, Savarino V, Peyrin‐Biroulet L, deCassan C, Dulbecco P, Baldissarro I, et al. Low serum trough levels are associated with post‐surgical recurrence in Crohn's disease patients undergoing prophylaxis with adalimumab. Digestive and Liver Disease 2014;46(11):1043‐6. - PubMed
Bodini 2015 {published data only}
-
- Bodini G, Savarino V, Marabotto E, Savarino E. Anti‐tumor necrosis factor antibodies for prevention of Crohn's disease recurrence after surgery: more than a hope. Clinical Gastroenterology and Hepatology 2015;13(10):1856. - PubMed
Bourreille 2005 {published data only}
-
- Bourreille A. Efficacy of azathioprine and its derivative, 6‐mercaptopurine in the prevention of post operative recurrent Crohn's disease [Efficacité de l’azathioprine et de son dérivé la 6‐mercaptopurine pour la prévention des rechutes post‐opératoires de maladie de Crohn]. Gastroentérologie Clinique et Biologique 2005;29(3):319‐21. [DOI: ]
De Cruz 2012 {published data only}
-
- Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Gorelik A, Liew D, et al. Adalimumab prevents post‐operative Crohn's disease recurrence, and is superior to thiopurines: early results from the POCER study. Journal of Gastroenterology and Hepatology 2012;4:100.
De Cruz 2013a {published data only}
-
- Cruz P, Kamm M, Hamilton A, Ritchie K, Gearry R (editors). Strategic timing of anti‐TNF therapy in postoperative Crohn's disease: comparison of routine use immediately postoperatively with selective use after demonstrated recurrence at 6 month endoscopy. Results from POCER. Journal of Gastroenterology and Hepatology 2013;28(Suppl 2):92.
De Cruz 2013b {published data only}
-
- Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Smoking is the key risk factor that doubles the risk of postoperative recurrence of Crohn's disease despite preventive drug treatment. Results from the POCER study. United European Gastroenterology Journal 2013;1:A95.
De Cruz 2013c {published data only}
-
- Cruz P, Kamm M, Hamilton A, Ritchie K, Krejany S, Gorelik A, et al. Strategic timing of anti‐TNF therapy in postoperative Crohn's disease: comparison of routine use immediately postoperatively with selective use after demonstrated recurrence at 6 month endoscopy: results from POCER. United European Gastroenterology Journal 2013;1:A16.
De Cruz 2015a {published data only}
-
- Cruz P, Kamm A, Hamilton KJ, Ritchie EO, Krejany A, Gorelik D, et al. Efficacy of thiopurines and adalimumab in preventing Crohn's disease recurrence in high‐risk patients ‐ a POCER study analysis. Alimentary Pharmacology & Therapeutics 2015;42(7):867‐79. - PubMed
De Cruz 2015b {published data only}
Doherty 2009 {published data only}
-
- Doherty GA, Cheifetz AS. Targeting TNF in postoperative recurrence of Crohn's disease: can we extinguish the fire before it starts?. Inflammatory Bowel Diseases 2009;15(12):1925‐6. - PubMed
Dumois 2001 {published data only}
-
- Dumois RA, Herrera JL. Can postoperative relapse of Crohn's disease be prevented?. American Journal of Gastroenterology 2001;96(1):249. - PubMed
Ewe 1976 {published data only}
-
- Ewe K, Holtermuller KH, Baas U, Eckardt V, Kreig H, Kutzner J, et al. Prophylaxis after resection because of Crohn’s disease by Salazosulfapyridin (Azulfidine). A double‐blind study [Rezidivprophylaxenach darmresektion wegen Morbus Crohn durch Salazosulfapyridin (Azulfidine). Eine doppelblindstudie]. Verhandlungen der Deutschen Gesellschaft fur Innere Medizin 1976;82(Pt 1):930‐2. - PubMed
Ewe 1980 {published data only}
-
- Ewe K, Herfarth C, Malchow H. Surgical and internal medicine therapy study of the postoperative prevention of recurrence in Crohn's disease ‐ completion of a partly randomized study. Verhandlungen der Deutschen Gesellschaft fur Innere Medizin 1980;86:1327‐37. - PubMed
Ewe 1981 {published data only}
-
- Ewe K. Effectiveness of Azulfidine/Salazopyrin in the postoperative prevention of recurrence in Crohn disease [Wirksamkeit von Azulfidine/Salazopyrin zur postoperativen rezidivprophylaxe bei Morbus Crohn]. Zeitschrift fur Gastroenterologie ‐ Verhandlungsband 1981;19:41‐4. - PubMed
Ewe 1984 {published data only}
-
- Ewe K, Malchow H, Herfarth C. Radical operation and recurrence prevention with azulfidine in Crohn disease: a prospective multicenter study ‐ initial results. Langenbecks Archiv fur Chirurgie 1984;364:427‐30. - PubMed
Ferrante 2014 {published data only}
-
- Ferrante M, Papamichael K, Duricova D, D'Haens GH, Vermeire S, Archavlis EJ, et al. Systematic versus endoscopy‐driven treatment with azathioprine to prevent postoperative ileal Crohn's disease recurrence: interim results from a randomized, multicenter trial. Gastroenterology 2014;146(5 Suppl 1):S‐592. - PubMed
Ford 2010 {published data only}
-
- Ford AC. Efficacy of azathioprine versus mesalazine in postoperative Crohn's disease. Gut 2010;59(12):1731‐2. - PubMed
Herfarth 2014 {published data only}
-
- Herfarth HH. Anti‐tumor necrosis factor therapy to prevent Crohn's disease recurrence after surgery. Clinical Gastroenterology and Hepatology 2014;12(9):1503‐6. - PubMed
Kamm 2014a {published data only}
-
- Kamm MA, Cruz PP, Wright EK, Hamilton AL, Ritchie KJ, Krejany EO, et al. Optimising post‐operative Crohn's disease management: best drug therapy alone versus colonoscopic monitoring with treatment step‐up. The POCER study. Gastroenterology 2014;1:S164.
Kamm 2014b {published data only}
-
- Kamm MA, Cruz PP, Wright EK, Hamilton AL, Ritchie KJ, Krejany EO. Optimising post‐operative Crohn's disease management: best drug therapy alone versus endoscopic monitoring, disease evolution, and faecal calprotectin monitoring. The POCER study. Journal of Crohn's and Colitis 2014;8:S13.
Kennedy 2015 {published data only}
-
- Kennedy NA, Ennis H, Gaya DR, Mowat C, Arnott IDR. Interobserver agreement in assessment of Rutgeerts' score of endoscopic recurrence of ileal Crohn's disease: a substudy of the TOPPIC trial. Journal of Crohn's and Colitis 2015;9:S231‐2.
Liao 2009 {published data only}
-
- Liao NS, Ren JA, Fan CG, Wang GF, Zhao YZ, Li JS. Efficacy of polyglycosides of Tripterygium wilfordii in preventing postoperative recurrence of Crohn disease. Chinese Journal of Gastrointestinal Surgery 2009;12(2):167‐9. - PubMed
Manship 2015 {published data only}
-
- Manship TA, Ford AC. VSL#3 in postoperative Crohn's disease. Clinical Gastroenterology and Hepatology 2015;13(10):1855. - PubMed
Mardini 2005 {published data only}
-
- Mardini HE. Azathioprine and 6 Mercaptopurine in postoperative maintenance of Crohn's disease remission: is no evidence of effect an evidence of no effect? [3] (multiple letters). Gastroenterology 2005;128(1):246‐9. - PubMed
McLeod 1997 {published data only}
-
- McLeod RS, Wolff BG, Steinhart AH, Carryer PW, O'Rourke K, Andrews DF, et al. Risk and significance of endoscopic/radiological evidence of recurrent Crohn's disease. Gastroenterology 1997;113(6):1823‐7. - PubMed
NCT00074542 {published data only}
-
- NCT00074542. An efficacy and safety study of Omega‐3 free fatty acids (Epanova™) for the maintenance of symptomatic remission in subjects with Crohn's disease. clinicaltrials.gov/ct2/show/NCT00074542 (first received 16 December 2003).
NCT01190839 {published data only}
-
- NCT01190839. A multicenter trial comparing REMICADE (Infliximab) and placebo in the prevention of recurrence in Crohn's disease (CD) patients undergoing surgical resection who are at an increased risk of recurrence. clinicaltrials.gov/ct2/show/NCT01190839 (first received 30 August 2010).
NCT01696942 {published data only}
-
- NCT01696942. Cimzia versus mesalamine for Crohn's recurrence. clinicaltrials.gov/show/NCT01696942 (first received 2 October 2012).
NCT02247258 {published data only}
-
- NCT02247258. Azathioprine in the prevention of ileal Crohn's disease postoperative recurrence. clinicaltrials.gov/ct2/show/NCT02247258 (first received 23 September 2014).
NCT02255370 {published data only}
-
- NCT02255370. Curcumin associated with thiopurin in the prevention of post‐op recurrence in Crohn disease. clinicaltrials.gov/ct2/show/NCT02255370 (first received 2 October 2014).
NCT02997059 {published data only}
-
- NCT02997059. Effect of fluconazole on the levels of ASCA after surgical resection for Crohn's disease. clinicaltrials.gov/ct2/show/NCT02997059 (first received 19 December 2016).
Papamichael 2012 {published data only}
-
- Papamichael K, Lariou C, Mantzaris GJ. Adalimumab for the prevention and/or treatment of post‐operative recurrence of Crohn's disease: a prospective, two‐year, single center, pilot study. Journal of Crohn's and Colitis 2012;6(9):924‐31. - PubMed
Regueiro 2013 {published data only}
-
- Regueiro M, Baidoo L, Kip KE, Swoger JM, Binion DG, Hashash JG, et al. Infliximab maintenance beyond one year prevents postoperative Crohn's disease recurrence: long‐term follow‐up from the randomized controlled pilot study. Gastroenterology 2013;144(5 Suppl 1):S173.
Regueiro 2014 {published data only}
-
- Regueiro M, Kip KE, Baidoo L, Swoger JM, Schraut W. Postoperative therapy with infliximab prevents long‐term Crohn's disease recurrence. Clinical Gastroenterology and Hepatology 2014;12(9):1492‐502.e1. - PubMed
Reibetanz 2015 {published data only}
-
- Reibetanz J, Germer CT. Optimal management of Crohn's disease after intestinal resection. Der Chirurg; Zeitschrift fur alle Gebiete der Operativen Medizen 2015;86(11):1070. - PubMed
Ren 2013 {published data only}
-
- Ren J, Wu X, Liao N, Wang G, Fan C, Liu S, et al. Prevention of postoperative recurrence of Crohn's disease: Tripterygium wilfordii polyglycoside versus mesalazine. Journal of International Medical Research 2013;41(1):176‐87. - PubMed
Sandborn 2004 {published data only}
-
- Sandborn WJ, Feagan BG. The efficacy of azathioprine and 6‐mercaptopurine for the prevention of postoperative recurrence in patients with Crohn's disease remains uncertain. Gastroenterology 2004;127(3):990‐3. - PubMed
Steinhart 1992 {published data only}
-
- Steinhart AH, O'Rourke K, Wolff BG, McLeod RS. Application of a stopping rule based on total treatment failures: the postoperative Crohn's disease trial. Journal of Clinical Epidemiology 1992;45(5):495‐504. - PubMed
Tao 2009 {published data only}
-
- Tao QS, Ren JA, Ji ZL, Li JS, Wang XB, Jiang XH. Maintenance effect of polyglycosides of Tripterygium wilfordii on remission in postoperative Crohn disease. Chinese Journal of Gastrointestinal Surgery 2009;12(5):491‐3. - PubMed
Vera‐Mendoza 2017 {published data only}
-
- Vera‐Mendoza I, Domenech E, Taxonera C, Ruiz VV, Marin‐Jimenez I, Guardiola J, et al. Adalimumab vs azathioprine in the prevention of postoperative Crohn's disease recurrence. A GETECCU randomised trial. Journal of Crohn's and Colitis 2017;11(11):1293‐301. - PubMed
Wright 2014 {published data only}
-
- Wright EK, Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany S, et al. Intestinal resection in Crohn's disease is associated with significant and durable improvement in health related quality of life although to a lesser extent in women and smokers. Results from the POCER study. Gastroenterology 2014;156:S435.
Wright 2015 {published data only}
-
- Wright EK, Kamm MA, Cruz P, Hamilton AL, Ritchie K, Bell SJ, et al. Structured post‐operative treatment and monitoring to prevent Crohn's disease recurrence is cost effective. Results from the POCER study. Journal of Gastroenterology and Hepatology 2015;30:145.
Yamamoto 2009 {published data only}
-
- Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn's disease: a prospective pilot study. Inflammatory Bowel Diseases 2009;15(10):1460‐6. - PubMed
Yamamoto 2013 {published data only}
-
- Yamamoto T. Adalimumab or infliximab for the prevention or treatment of post‐operative recurrence in Crohn's disease. Journal of Crohn's and Colitis 2013;7(4):157. - PubMed
Zhu 2015 {published data only}
-
- Zhu W, Li Y, Gong J, Zuo L, Zhang W, Cao L, et al. Tripterygium wilfordii Hook. f. versus azathioprine for prevention of postoperative recurrence in patients with Crohn's disease: a randomized clinical trial. Digestive and Liver Disease 2015;47(1):14‐9. - PubMed
References to studies awaiting assessment
NCT00976690 {published data only}
-
- NCT00976690. Comparison azathioprine to mesalazine for the prevention of postoperative recurrence in the Crohn Disease (IMURELPOST). clinicaltrials.gov/ct2/show/NCT00976690 (first received 14 September 2009).
NCT01698970 {published data only}
-
- NCT01698970. Effect of the consumption of a probiotic strain on the prevention of post‐operative recurrence in Crohn's disease. clinicaltrials.gov/ct2/show/NCT01698970 (first received 3 October 2012).
References to ongoing studies
EUCTR2015‐000555‐24‐NL {published data only}
-
- EUCTR2015‐000555‐24‐NL. Randomized, placebo‐controlled, double‐blind, multicenter study to determine the effectiveness and safety of vedolizumab in prevention of recurrence of Crohn's disease of the mucosa in patients with surgical removal of the area between the small and the large bowel. clinicaltrialsregister.eu/ctr‐search/trial/2015‐000555‐24/NL (first received 2 October 2016).
NCT01015391 {published data only}
-
- NCT01015391. Efficacy study of T2 versus AZA to maintain clinical and endoscopic remission in postoperative Crohn's disease. clinicaltrials.gov/show/NCT01015391 (first received 18 November 2009).
NCT02834754 {published data only}
-
- NCT02834754. A randomized, double‐blind, placebo controlled study of vedolizumab for the prevention of post‐operative Crohn's disease recurrence. clinicaltrials.gov/show/NCT02834754 (first received 15 July 2016).
NCT03185611 {published data only}
-
- NCT03185611. Effectiveness of rifaximin combined with thiopurine on preventing postoperative recurrence in Crohn's disease. clinicaltrials.gov/show/NCT03185611 (first received 14 June 2017).
NCT03185624 {published data only}
-
- NCT03185624. Effectiveness of rifaximin on preventing postoperative recurrence in Crohn's disease. clinicaltrials.gov/ct2/show/NCT03185624 (first received 14 June 2017).
NCT03456752 {published data only}
-
- NCT03456752. Perioperative dexamethasone on postoperative outcome in IBD. clinicaltrials.gov/show/NCT03456752 (first received 7 March 2018).
NL6213 (NTR6385) {published data only}
-
- NL6213 (NTR6385). A trial to compare if the diseased area between the colon and the small bowel of patients with Crohn's disease stays free of disease after surgery between a group of patients whom receive no medication versus a group whom receive vedolizumab. https://www.trialregister.nl/trial/6213 (first received 24 April 2017).
Additional references
Achana 2013
-
- Achana FA, Cooper NJ, Dias S, Lu G, Rice SIC, Kendrick D, et al. Extending methods for investigating the relationship between treatment effect and baseline risk for pairwise meta‐analysis to network meta‐analysis. Statistics in Medicine 2013;32:752‐71. - PubMed
Akobeng 2016
Behm 2008
Bernell 2000
Borenstein 2009
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2009.
Chande 2015
CINeMA 2017 [Computer program]
-
- Institute of Social and Preventive Medicine, University of Bern. CINeMA: Confidence in Network Meta‐Analysis. Bern: University of Bern, accessed 9 February 2019.
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates Inc, 1988.
Colonna 1994
-
- Colonna T, Korelitz BI. The role of leukopenia in the 6‐mercaptopurine induced remission of refractory Crohn's disease. American Journal of Gastroenterology 1994;89:362‐6. - PubMed
Cooper 2009
-
- Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between‐study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non‐rheumatic atrial fibrillation. Statistics in Medicine 2009;28:1861‐81. - PubMed
Cui 2004
Di Sario 2016
-
- Sario A, Bendia E, Schiadà L, Sassaroli P, Benedetti A. Biologic drugs in Crohn's disease and ulcerative colitis: safety profile. Current Drug Safety 2016;11:55‐61. - PubMed
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistical Methodology 2010;29:932‐44. - PubMed
Egger 2001
-
- Egger M, Davey‐Smith G, Altman D (editors). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001.
FDA 2018
-
- US Food, Drug Administration. Code of Federal Regulations Title 21.:21CFR312.32. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?f... (accessed 10 January 2018).
Gionchetti 2017
-
- Gionchetti P, Dignass A, Danese S, Dias FJM, Rogler G, Lakatos PL. ECCO; 3rd European evidence‐based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: Surgical management and special situations. Journal of Crohn's and Colitis 2017;11:135‐49. - PubMed
Gjuladin‐Hellon 2019a
Gjuladin‐Hellon 2019b
Gklavas 2017
Gordon 2017
GRADEpro 2015 [Computer program]
-
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University, 2015 (developed by Evidence Prime, Inc.).
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6: rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. - PubMed
Hafraoui 2002
-
- Hafraoui S, Dewit O, Marteau P, Cosnes J, Colombel JF, Modigliani R, et al. Mycophenolate mofetil in refractory Crohn's disease after failure of treatments by azathioprine or methotrexate [Le mycophénolate mofétil dans les formes chroniques actives de la maladie de Crohn après échec de lazathioprine ou du méthotrexate]. Gastroenterologie Clinique et Biologique 2002;26:17‐22. - PubMed
Hanauer 2001
-
- Hanauer SB, Sandborn W. Management of Crohn’s disease in adults. American Journal of Gastroenterology 2001;96:635‐43. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jansen 2014
-
- Jansen JP, Trikalinos T, Cappelleri JC, Daw J, Andes S, Eldessouki R, et al. Indirect treatment comparison/network meta‐analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR‐AMCP‐NPC Good Practice Task Force report. Value Health 2014;17:157‐73. - PubMed
Kim 2017
-
- Kim A, Roberts C, Feagan B, Banerjee R, Bemelman W, Bodger K. Developing a standard set of patient‐centred outcomes for inflammatory bowel disease ‐ an international, cross‐disciplinary consensus. Journal of Crohn's and Colitis 2017;12:408‐18. - PubMed
Levin 2016
-
- Levin AD, Wildenberg ME, Brink GR. Mechanism of action of anti‐TNF therapy in inflammatory bowel disease. Journal of Crohn's and Colitis 2016;10:989‐97. - PubMed
Lichtenstein 2018
-
- Lichtenstein G, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. Management of Crohn’s disease in adults. American Journal of Gastroenterology 2018;113:481‐517. - PubMed
Lunn 2000
-
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS — a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing 2000;10:325‐37.
Ma 2017
Mbuagbaw 2017
NICE 2012
NICE 2019
-
- National Institute of Health and Care Excellence. Crohn’s disease: management [NG129]. www.nice.org.uk/guidance/ng129/evidence/postsurgical‐maintenance‐of‐remi... (accessed 12 June 2019).
Norman 2018
Orlando 2014
-
- Orlando A, Mocciaro F, Renna S, Scimeca D, Rispo A, Scribano ML, et al. Early post‐operative endoscopic recurrence in Crohn's disease patients: data from an Italian Group for the study of inflammatory bowel disease (IG‐IBD) study on a large prospective multicenter cohort. Journal of Crohn's and Colitis 2014;8(10):1217‐21. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistical Methodology 1998;17:2815‐34. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutgeerts 2002
Salanti 2014
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Spiegelhalter 2002
-
- Spiegelhalter DJ, Best NG, Carlin BP, Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B 2002;64:583‐639.
Spiegelhalter 2004
-
- Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health‐Care Evaluation. Chichester, West Sussex, UK: John Wiley and Sons Ltd, 2004.
Stata 2017 [Computer program]
-
- StataCorp LLC. Stata Statistical Software. Version Release 15. College Station, TX: StataCorp LLC, 2017.
Steinhart 2003
Sutton 2000
-
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta‐analysis in medical research. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2000.
Svartz 1942
-
- Svartz N. Salazopyrin, a new sulfanilamide preparation: A. Therapeutic results in rheumatic polyarthritis. B. Therapeutic results in ulcerative colitis. C. Toxic manifestations in treatment with sulfanilamide preparation. Acta Medica Scandinavica 1942;110:557‐90.
Warren 2014
-
- Warren FC, Abrams KR, Sutton AJ. Hierarchical network meta‐analysis models to address sparsity of events and differing treatment classifications with regard to adverse outcomes. Statistics in Medicine 2014;33:2449‐61. - PubMed
Welton 2012
-
- Welton NJ, Sutton AJ, Cooper NJ, Abrams KR, Ades AE. Evidence synthesis in decision making in healthcare. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2012.
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

