Vemurafenib for Refractory Multisystem Langerhans Cell Histiocytosis in Children: An International Observational Study
- PMID: 31513482
- PMCID: PMC6823889
- DOI: 10.1200/JCO.19.00456
Vemurafenib for Refractory Multisystem Langerhans Cell Histiocytosis in Children: An International Observational Study
Abstract
Purpose: Off-label use of vemurafenib (VMF) to treat BRAFV600E mutation-positive, refractory, childhood Langerhans cell histiocytosis (LCH) was evaluated.
Patients and methods: Fifty-four patients from 12 countries took VMF 20 mg/kg/d. They were classified according to risk organ involvement: liver, spleen, and/or blood cytopenia. The main evaluation criteria were adverse events (Common Terminology Criteria for Adverse Events [version 4.3]) and therapeutic responses according to Disease Activity Score.
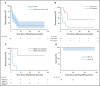
Results: LCH extent was distributed as follows: 44 with positive and 10 with negative risk organ involvement. Median age at diagnosis was 0.9 years (range, 0.1 to 6.5 years). Median age at VMF initiation was 1.8 years (range, 0.18 to 14 years), with a median follow-up of 22 months (range, 4.3 to 57 months), whereas median treatment duration was 13.9 months (for 855 patient-months). At 8 weeks, 38 complete responses and 16 partial responses had been achieved, with the median Disease Activity Score decreasing from 7 at diagnosis to 0 (P < .001). Skin rash, the most frequent adverse event, affected 74% of patients. No secondary skin cancer was observed. Therapeutic plasma VMF concentrations (range, 10 to 20 mg/L) seemed to be safe and effective. VMF discontinuation for 30 patients led to 24 LCH reactivations. The blood BRAFV600E allele load, assessed as circulating cell-free DNA, decreased after starting VMF but remained positive (median, 3.6% at diagnosis, and 1.6% during VMF treatment; P < .001) and was associated with a higher risk of reactivation at VMF discontinuation. None of the various empirical therapies (hematopoietic stem-cell transplantation, cladribine and cytarabine, anti-MEK agent, vinblastine, etc) used for maintenance could eradicate the BRAFV600E clone.
Conclusion: VMF seemed safe and effective in children with refractory BRAFV600E-positive LCH. Additional studies are needed to find effective maintenance therapy approaches.
Figures


References
-
- Haroche J, Cohen-Aubart F, Rollins BJ, et al. Histiocytoses: Emerging neoplasia behind inflammation. Lancet Oncol. 2017;18:e113–e125. - PubMed
-
- Rigaud C, Barkaoui MA, Thomas C, et al. Langerhans cell histiocytosis: Therapeutic strategy and outcome in a 30-year nationwide cohort of 1478 patients under 18 years of age. Br J Haematol. 2016;174:887–898. - PubMed
-
- Minkov M, Grois N, Heitger A, et al. Response to initial treatment of multisystem Langerhans cell histiocytosis: An important prognostic indicator. Med Pediatr Oncol. 2002;39:581–585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

