Loss-of-function variants in myocardin cause congenital megabladder in humans and mice
- PMID: 31513549
- PMCID: PMC6877301
- DOI: 10.1172/JCI128545
Loss-of-function variants in myocardin cause congenital megabladder in humans and mice
Abstract
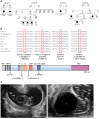
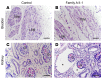
Myocardin (MYOCD) is the founding member of a class of transcriptional coactivators that bind the serum-response factor to activate gene expression programs critical in smooth muscle (SM) and cardiac muscle development. Insights into the molecular functions of MYOCD have been obtained from cell culture studies, and to date, knowledge about in vivo roles of MYOCD comes exclusively from experimental animals. Here, we defined an often lethal congenital human disease associated with inheritance of pathogenic MYOCD variants. This disease manifested as a massively dilated urinary bladder, or megabladder, with disrupted SM in its wall. We provided evidence that monoallelic loss-of-function variants in MYOCD caused congenital megabladder in males only, whereas biallelic variants were associated with disease in both sexes, with a phenotype additionally involving the cardiovascular system. These results were supported by cosegregation of MYOCD variants with the phenotype in 4 unrelated families by in vitro transactivation studies in which pathogenic variants resulted in abrogated SM gene expression and by the finding of megabladder in 2 distinct mouse models with reduced Myocd activity. In conclusion, we have demonstrated that variants in MYOCD result in human disease, and the collective findings highlight a vital role for MYOCD in mammalian organogenesis.
Keywords: Genetic diseases; Molecular genetics; Muscle Biology; Urology.
Conflict of interest statement
Figures
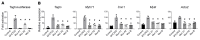

References
-
- Wiesel A, Queisser-Luft A, Clementi M, Bianca S, Stoll C; EUROSCAN Study Group Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: an analysis of 709,030 births in 12 European countries. Eur J Med Genet. 2005;48(2):131–44. doi: 10.1016/j.ejmg.2005.02.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

