Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial
- PMID: 31513665
- PMCID: PMC6742232
- DOI: 10.1371/journal.pmed.1002901
Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial
Abstract
Background: The inflammatory contribution to type 2 diabetes (T2D) has suggested new therapeutic targets using biologic drugs designed for rheumatoid arthritis (RA). On this basis, we aimed at investigating whether interleukin-1 (IL-1) inhibition with anakinra, a recombinant human IL-1 receptor antagonist, could improve both glycaemic and inflammatory parameters in participants with RA and T2D compared with tumour necrosis factor (TNF) inhibitors (TNFis).
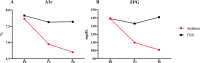
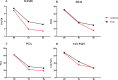
Methods and findings: This study, designed as a multicentre, open-label, randomised controlled trial, enrolled participants, followed up for 6 months, with RA and T2D in 12 Italian rheumatologic units between 2013 and 2016. Participants were randomised to anakinra or to a TNFi (i.e., adalimumab, certolizumab pegol, etanercept, infliximab, or golimumab), and the primary end point was the change in percentage of glycated haemoglobin (HbA1c%) (EudraCT: 2012-005370-62 ClinicalTrial.gov: NCT02236481). In total, 41 participants with RA and T2D were randomised, and 39 eligible participants were treated (age 62.72 ± 9.97 years, 74.4% female sex). The majority of participants had seropositive RA disease (rheumatoid factor and/or anticyclic citrullinated peptide antibody [ACPA] 70.2%) with active disease (Disease Activity Score-28 [DAS28]: 5.54 ± 1.03; C-reactive protein 11.84 ± 9.67 mg/L, respectively). All participants had T2D (HbA1c%: 7.77 ± 0.70, fasting plasma glucose: 139.13 ± 42.17 mg). When all the enrolled participants reached 6 months of follow-up, the important crude difference in the main end point, confirmed by an unplanned ad interim analysis showing the significant effects of anakinra, which were not observed in the other group, led to the study being stopped for early benefit. Participants in the anakinra group had a significant reduction of HbA1c%, in an unadjusted linear mixed model, after 3 months (β: -0.85, p < 0.001, 95% CI -1.28 to -0.42) and 6 months (β: -1.05, p < 0.001, 95% CI -1.50 to -0.59). Similar results were observed adjusting the model for relevant RA and T2D clinical confounders (male sex, age, ACPA positivity, use of corticosteroids, RA duration, T2D duration, use of oral antidiabetic drug, body mass index [BMI]) after 3 months (β: -1.04, p < 0.001, 95% CI -1.52 to -0.55) and 6 months (β: -1.24, p < 0.001, 95% CI -1.75 to -0.72). Participants in the TNFi group had a nonsignificant slight decrease of HbA1c%. Assuming the success threshold to be HbA1c% ≤ 7, we considered an absolute risk reduction (ARR) = 0.42 (experimental event rate = 0.54, control event rate = 0.12); thus, we estimated, rounding up, a number needed to treat (NNT) = 3. Concerning RA, a progressive reduction of disease activity was observed in both groups. No severe adverse events, hypoglycaemic episodes, or deaths were observed. Urticarial lesions at the injection site led to discontinuation in 4 (18%) anakinra-treated participants. Additionally, we observed nonsevere infections, including influenza, nasopharyngitis, upper respiratory tract infection, urinary tract infection, and diarrhoea in both groups. Our study has some limitations, including open-label design and previously unplanned ad interim analysis, small size, lack of some laboratory evaluations, and ongoing use of other drugs.
Conclusions: In this study, we observed an apparent benefit of IL-1 inhibition in participants with RA and T2D, reaching the therapeutic targets of both diseases. Our results suggest the concept that IL-1 inhibition may be considered a targeted treatment for RA and T2D.
Trial registration: The trial is registered with EU Clinical Trials Register, EudraCT Number: 2012-005370-62 and with ClinicalTrial.gov, number NCT02236481.
Conflict of interest statement
The authors have declared that no competing interests exist for this work.
Figures
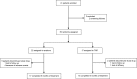
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

