Peramivir for Influenza A and B Viral Infections: A Pharmacokinetic Case Series
- PMID: 31514223
- PMCID: PMC7167779
- DOI: 10.1002/phar.2330
Peramivir for Influenza A and B Viral Infections: A Pharmacokinetic Case Series
Abstract
Objective: To describe the peramivir (PRV) pharmacokinetics in critically ill children treated for influenza A or B viral infections.
Design: Retrospective electronic medical record review of prospectively collected data from critically ill children receiving peramivir for influenza A or B viral infections in the pediatric intensive care unit (PICU).
Setting: A 189-bed, freestanding children's tertiary care teaching hospital in Philadelphia, PA.
Patients: Critically ill children admitted to the PICU who were infected with influenza between January 1, 2016 and March 31, 2018.
Interventions: None.
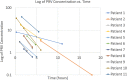
Results: Eleven patients, two females (18%) and nine males (82%), accounted for 24 peramivir samples for therapeutic drug management. The median age was 5 years (interquartile range 1.5-6.5 yrs) with a median weight of 16.4 kg (interquartile range 14-24 kg). Ten (91%) patients demonstrated a larger volume of distribution, 11 (100%) patients demonstrated an increase in clearance, and 11 (100%) patients demonstrated a shorter half-life estimate as compared with the package insert and previous pediatric trial data for peramivir. Eight (73%) patients tested positive for a strain of influenza A and 3 (27%) patients tested positive for influenza B; 4 of 11 (36%) patients tested positive for multiple viruses. All patients had adjustments made to their dosing interval to a more frequent interval. Ten (91%) patients were adjusted to an every-12-hour regimen and 1 (9%) patient was adjusted to an every-8-hour regimen. No adverse events were associated with peramivir treatment.
Conclusion: The pharmacokinetics of PRV demonstrated in this PICU cohort differs in comparison to healthy pediatric and adult patients, and alterations to dosing regimens may be needed in PICU patients to achieve pharmacodynamic exposures. Additional investigations in the PICU population are needed to confirm these findings.
Keywords: influenza; pediatric; peramivir; pharmacodynamic; pharmacokinetics.
© 2019 Pharmacotherapy Publications, Inc.
Figures
References
-
- Peramivir (Rapivab®) [package insert]. Durham, NC: BioCryst Pharmaceuticals; December 2014.
-
- Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta‐lactam antibiotic doses sufficient for critically ill patients? Clinical Infect Dis 2014;58(8):1072–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical