Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety
- PMID: 31514313
- PMCID: PMC6767014
- DOI: 10.3390/molecules24183302
Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety
Abstract
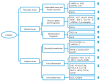
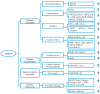
This review aimed to provide a general view of catalpol in protection against diabetes and diabetic complications, as well as its pharmacokinetics and safety concerns. The following databases were consulted with the retrieval of more than 100 publications through June 2019: PubMed, Chinese National Knowledge Infrastructure, WanFang Data, and web of science. Catalpol exerts an anti-diabetic effect in different animal models with an oral dosage ranging from 2.5 to 200 mg/kg in rats and 10 to 200 mg/kg in mice. Besides, catalpol may prevent the development of diabetic complications in kidney, heart, central nervous system, and bone. The underlying mechanism may be associated with an inhibition of inflammation, oxidative stress, and apoptosis through modulation of various cellular signaling, such as AMPK/PI3K/Akt, PPAR/ACC, JNK/NF-κB, and AGE/RAGE/NOX4 signaling pathways, as well as PKCγ and Cav-1 expression. The pharmacokinetic profile reveals that catalpol could pass the blood-brain barrier and has a potential to be orally administrated. Taken together, catalpol is a well-tolerated natural compound with promising pharmacological actions in protection against diabetes and diabetic complications via multi-targets, offering a novel scaffold for the development of anti-diabetic drug candidate. Further prospective and well-designed clinical trials will shed light on the potential of clinical usage of catalpol.
Keywords: catalpol; diabetes; diabetic complications; pharmacokinetic; pharmacology; safety.
Conflict of interest statement
The authors declare no conflict of interest regarding the publication of this paper. The funding agencies have no roles in study design, data analysis, drafting, and submitting the article.
Figures




References
-
- Søren D. Biosynthesis of catalpol. Phytochemistry. 1994;35:1187–1189.
-
- Tang W.C., Eisenbrand G. Chinese Drugs of Plant Origin. Springer; Berlin/Heidelberg, Germany: 1992. - DOI
-
- Li H.W., Meng X.L. Research progress on chemical constituents and pharmacological activities of Rehmannia glutinosa. Drug Eval. Res. 2015;38:218–228.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

