Deficiency of Mitochondrial Aspartate-Glutamate Carrier 1 Leads to Oligodendrocyte Precursor Cell Proliferation Defects Both In Vitro and In Vivo
- PMID: 31514314
- PMCID: PMC6769484
- DOI: 10.3390/ijms20184486
Deficiency of Mitochondrial Aspartate-Glutamate Carrier 1 Leads to Oligodendrocyte Precursor Cell Proliferation Defects Both In Vitro and In Vivo
Abstract
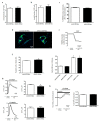
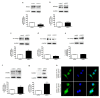
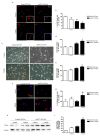
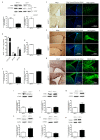
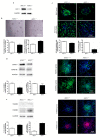
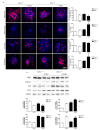
Aspartate-Glutamate Carrier 1 (AGC1) deficiency is a rare neurological disease caused by mutations in the solute carrier family 25, member 12 (SLC25A12) gene, encoding for the mitochondrial aspartate-glutamate carrier isoform 1 (AGC1), a component of the malate-aspartate NADH shuttle (MAS), expressed in excitable tissues only. AGC1 deficiency patients are children showing severe hypotonia, arrested psychomotor development, seizures and global hypomyelination. While the effect of AGC1 deficiency in neurons and neuronal function has been deeply studied, little is known about oligodendrocytes and their precursors, the brain cells involved in myelination. Here we studied the effect of AGC1 down-regulation on oligodendrocyte precursor cells (OPCs), using both in vitro and in vivo mouse disease models. In the cell model, we showed that a reduced expression of AGC1 induces a deficit of OPC proliferation leading to their spontaneous and precocious differentiation into oligodendrocytes. Interestingly, this effect seems to be related to a dysregulation in the expression of trophic factors and receptors involved in OPC proliferation/differentiation, such as Platelet-Derived Growth Factor α (PDGFα) and Transforming Growth Factor βs (TGFβs). We also confirmed the OPC reduction in vivo in AGC1-deficent mice, as well as a proliferation deficit in neurospheres from the Subventricular Zone (SVZ) of these animals, thus indicating that AGC1 reduction could affect the proliferation of different brain precursor cells. These data clearly show that AGC1 impairment alters myelination not only by acting on N-acetyl-aspartate production in neurons but also on OPC proliferation and suggest new potential therapeutic targets for the treatment of AGC1 deficiency.
Keywords: AGC1 deficiency; growth factors; mitochondrial disease; mouse model; subventricular zone.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Transcriptional and metabolic effects of aspartate-glutamate carrier isoform 1 (AGC1) downregulation in mouse oligodendrocyte precursor cells (OPCs).Cell Mol Biol Lett. 2024 Mar 29;29(1):44. doi: 10.1186/s11658-024-00563-z. Cell Mol Biol Lett. 2024. PMID: 38553684 Free PMC article.
-
AGC1 Deficiency: Pathology and Molecular and Cellular Mechanisms of the Disease.Int J Mol Sci. 2022 Jan 4;23(1):528. doi: 10.3390/ijms23010528. Int J Mol Sci. 2022. PMID: 35008954 Free PMC article. Review.
-
Down-regulation of the mitochondrial aspartate-glutamate carrier isoform 1 AGC1 inhibits proliferation and N-acetylaspartate synthesis in Neuro2A cells.Biochim Biophys Acta Mol Basis Dis. 2017 Jun;1863(6):1422-1435. doi: 10.1016/j.bbadis.2017.02.022. Epub 2017 Feb 21. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28235644
-
ARALAR/AGC1 deficiency, a neurodevelopmental disorder with severe impairment of neuronal mitochondrial respiration, does not produce a primary increase in brain lactate.J Neurochem. 2017 Jul;142(1):132-139. doi: 10.1111/jnc.14047. Epub 2017 May 22. J Neurochem. 2017. PMID: 28429368
-
The mitochondrial aspartate/glutamate carrier AGC1 and calcium homeostasis: physiological links and abnormalities in autism.Mol Neurobiol. 2011 Aug;44(1):83-92. doi: 10.1007/s12035-011-8192-2. Epub 2011 Jun 21. Mol Neurobiol. 2011. PMID: 21691713 Review.
Cited by
-
The role of glutamate and glutamine metabolism and related transporters in nerve cells.CNS Neurosci Ther. 2024 Feb;30(2):e14617. doi: 10.1111/cns.14617. CNS Neurosci Ther. 2024. PMID: 38358002 Free PMC article. Review.
-
Transcriptional and metabolic effects of aspartate-glutamate carrier isoform 1 (AGC1) downregulation in mouse oligodendrocyte precursor cells (OPCs).Cell Mol Biol Lett. 2024 Mar 29;29(1):44. doi: 10.1186/s11658-024-00563-z. Cell Mol Biol Lett. 2024. PMID: 38553684 Free PMC article.
-
AGC1 Deficiency: Pathology and Molecular and Cellular Mechanisms of the Disease.Int J Mol Sci. 2022 Jan 4;23(1):528. doi: 10.3390/ijms23010528. Int J Mol Sci. 2022. PMID: 35008954 Free PMC article. Review.
-
Calcium signaling in mitochondrial intermembrane space.Biochem Soc Trans. 2024 Oct 30;52(5):2215-2229. doi: 10.1042/BST20240319. Biochem Soc Trans. 2024. PMID: 39392359 Review.
-
Glutamate Carrier Involvement in Mitochondrial Dysfunctioning in the Brain White Matter.Front Mol Biosci. 2020 Jul 21;7:151. doi: 10.3389/fmolb.2020.00151. eCollection 2020. Front Mol Biosci. 2020. PMID: 32793632 Free PMC article.
References
-
- Indiveri C., Krämer R., Palmieri F. Reconstitution of the malate/aspartate shuttle from mitochondria. J. Boil. Chem. 1987;262:15979–15983. - PubMed
-
- Palmieri L., Pardo B., Lasorsa F.M., del Arco A., Kobayashi K., Iijima M., Runswick M.J., Walker J.E., Saheki T., Satrústegui J., et al. Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

