Towards Understanding Non-Infectious Growth-Rate Retardation in Growing Pigs
- PMID: 31514421
- PMCID: PMC6789591
- DOI: 10.3390/proteomes7030031
Towards Understanding Non-Infectious Growth-Rate Retardation in Growing Pigs
Abstract
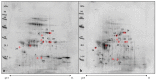
For growth-rate retardation in commercial growing pigs suffering from non-infectious diseases, no biomarker is available for early detection and prevention of the condition or for the diagnosis of affected animals. The point in question is that the underlying pathological pathway of the condition is still unknown and multiple nutritional or management issues could be the cause of the disease. Common health status markers such as acute phase proteins, adenosine deaminase activity or total antioxidant capacity did not show any alteration in the saliva of animals with growth-rate retardation, so other pathways should be affected. The present study investigates saliva samples from animals with the same commercial crossbreed, sex and age, comparing control pigs and pigs with growth-rate retardation. A proteomics approach based on two-dimensional gel electrophoresis including mass spectrometry together with validation experiments was applied for the search of proteins that could help understand disease mechanisms and be used for early disease detection. Two proteins were detected as possible markers of growth-rate retardation, specifically S100A12 and carbonic anhydrase VI. A decrease in innate immune response was confirmed in pigs with growth-rate retardation, however further studies should be necessary to understand the role of the different CA VI proteoforms observed.
Keywords: biomarker detection; gel-based proteomics; growth-rate retardation; pig; saliva.
Conflict of interest statement
The authors declare no conflict of interest
Figures



References
-
- Caravaca Rodríguez F.P., CastelGenís J.M., Guzman Guerrero J.L., Delgado Pertíñez M., Mena Guerrero Y., Alcalde Aldea M.J., González Redondo P., editors. Bases de la Producción Animal. Universidad de Sevilla and Universidad de Córdoba; Sevilla, Spain: 2005. Bases fisiológicas del crecimiento.
-
- Vázquez-Gómez M., García-Contreras C., Torres-Rovira L., Pesantez J.L., González-Añover P., Gómez-Fidalgo E., Sánchez-Sánchez E., Ovilo C., Isabel B., Astiz S., et al. Polyphenols and IUGR pregnancies: Maternal hydroxyl tyrosol supplementation improves prenatal and early-postnatal growth and metabolism of theoffspring. PLoS ONE. 2017;12:e0177593. doi: 10.1371/journal.pone.0177593. - DOI - PMC - PubMed
-
- Thomson J.R., Friendship R.M. Digestive System. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., editors. Diseases of Swine. 10th ed. John Wiley and Sons; London, UK: 2012. pp. 199–226.
-
- Pierozan C.R., Agostini P.S., Gasa J., Novais A.K., Dias C.P., Santos R.S.K., Pereira M., Nagi J.G.J., Alves B., Silva C.A. Factors affecting the daily feed intake and feed conversion ratio of pigs in grow-finishing units: The case of a company. Porcine Health Manag. 2016;2:7. doi: 10.1186/s40813-016-0023-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

