Targeting Autophagy by MPT0L145, a Highly Potent PIK3C3 Inhibitor, Provides Synergistic Interaction to Targeted or Chemotherapeutic Agents in Cancer Cells
- PMID: 31514441
- PMCID: PMC6770340
- DOI: 10.3390/cancers11091345
Targeting Autophagy by MPT0L145, a Highly Potent PIK3C3 Inhibitor, Provides Synergistic Interaction to Targeted or Chemotherapeutic Agents in Cancer Cells
Abstract
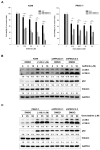
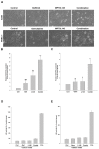
Anticancer therapies reportedly promote pro-survival autophagy in cancer cells that confers drug resistance, rationalizing the concept to combine autophagy inhibitors to increase their therapeutic potential. We previously identified that MPT0L145 is a PIK3C3/FGFR inhibitor that not only increases autophagosome formation due to fibroblast growth factor receptor (FGFR) inhibition but also perturbs autophagic flux via PIK3C3 inhibition in bladder cancer cells harboring FGFR activation. In this study, we hypothesized that combined-use of MPT0L145 with agents that induce pro-survival autophagy may provide synthetic lethality in cancer cells without FGFR activation. The results showed that MPT0L145 synergistically sensitizes anticancer effects of gefitinib and gemcitabine in non-small cell lung cancer A549 cells and pancreatic cancer PANC-1 cells, respectively. Mechanistically, drug combination increased incomplete autophagy due to impaired PIK3C3 function by MPT0L145 as evidenced by p62 accumulation and no additional apoptotic cell death was observed. Meanwhile, drug combination perturbed survival pathways and increased vacuolization and ROS production in cancer cells. In conclusion, the data suggest that halting pro-survival autophagy by targeting PIK3C3 with MPT0L145 significantly sensitizes cancer cells to targeted or chemotherapeutic agents, fostering rational combination strategies for cancer therapy in the future.
Keywords: PIK3C3; autophagy; gefitinib; gemcitabine; synergism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

