Antimicrobial use policy change in pre-weaned dairy calves and its impact on antimicrobial resistance in commensal Escherichia coli: a cross sectional and ecological study
- PMID: 31514734
- PMCID: PMC6739941
- DOI: 10.1186/s12866-019-1576-6
Antimicrobial use policy change in pre-weaned dairy calves and its impact on antimicrobial resistance in commensal Escherichia coli: a cross sectional and ecological study
Abstract
Background: This study is based on data collected to investigate the relation of peri-parturient events (colostrum quality, passive transfer of immunity, calving difficulty) on calf health and antimicrobial use. A component of the study was to provide feedback to farm management to identify calves at risk for disease and promote antimicrobial stewardship. At the start of the study (May 2016), a combination of enrofloxacin, penicillin, and sulfamethoxazole was the first treatment given to clinically abnormal calves. Based on feedback and interaction between study investigators, farm management and consulting veterinarians, a new policy was implemented to reduce antimicrobial use in calves. In August, the first treatment was changed to a combination of ampicillin and sulfamethoxazole. In September, the first treatment was reduced to only sulfamethoxazole. We investigated the effects of these policy changes in antimicrobial use on resistance in commensal Escherichia coli.
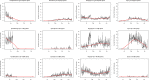
Results: We enrolled 4301 calves at birth and documented antimicrobial use until weaning. Most calves (99.4%) received antimicrobials and 70.4% received a total of 2-4 treatments. Antimicrobial use was more intense in younger calves (≤ 28 days) relative to older calves. We isolated 544 E. coli from fecal samples obtained from 132 calves. We determined resistance to 12 antimicrobials and 85% of the isolates were resistant to at least 3 antimicrobial classes. We performed latent class analysis to identify underlying unique classes where isolates shared resistance patterns and selected a solution with 4 classes. The least resistant class had isolates that were mainly resistant to only tetracycline and sulfisoxazole. The other 3 classes comprised isolates with resistance to ampicillin, chloramphenicol, aminoglycosides, sulfonamides, tetracycline, in addition to either ceftiofur; or nalidixic acid; or ciprofloxacin plus nalidixic acid and ceftiofur. Overall, E coli from younger calves and calves that received multiple treatments were more likely to have extensive resistance including resistance to fluoroquinolones and ceftiofur. In general, there was a declining trend in resistance to most antimicrobials during and after policy changes were implemented, except for ampicillin, ciprofloxacin, ceftiofur and gentamicin.
Conclusions: Information feedback to farms can influence farm managers to reduce antimicrobial use and this can change endemic farm resistance patterns.
Keywords: Antimicrobial resistance; Antimicrobial use; Feedback; Policy change; Pre-weaned dairy calves.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Tang KL, Caffrey NP, Nobrega DB, Cork SC, Ronksley PE, Barkema HW, et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 2017;1:e316–e327. doi: 10.1016/S2542-5196(17)30141-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

