TRPP2 dysfunction decreases ATP-evoked calcium, induces cell aggregation and stimulates proliferation in T lymphocytes
- PMID: 31514750
- PMCID: PMC6743124
- DOI: 10.1186/s12882-019-1540-6
TRPP2 dysfunction decreases ATP-evoked calcium, induces cell aggregation and stimulates proliferation in T lymphocytes
Abstract
Background: Autosomal dominant polycystic kidney disease (ADPKD) is mainly characterised by the development and enlargement of renal cysts that lead to end-stage renal disease (ESRD) in adult patients. Other clinical manifestations of this pathology include hypertension, haematuria, abdominal pain, cardiovascular system alterations and intracranial aneurysms. ADPKD is linked to mutations in either PKD1 or PKD2 that codifies polycystin-1 (PC1) and polycystin-2 (PC2 or TRPP2), respectively. PC1 and TRPP2 are membrane proteins that function as receptor-channel elements able to regulate calcium homeostasis. The function of polycystins has been mainly studied in kidney cells; but the role of these proteins in T lymphocytes is not well defined.
Methods: T lymphocytes were produced from ADPKD1 and ADPKD2 patients as well as from non-ADPKD subjects undergoing renal replacement therapy (RRT) and healthy controls. Protein expression and phosphorylation levels were analysed by western blotting, cell proliferation was calculated by direct counting using trypan blue assay and intracellular calcium concentration was measured by Fura-2 method.
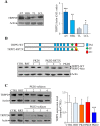
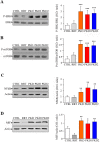
Results: PKD2 mutations lead to the significant reduction of TRPP2 expression in T lymphocytes derived from ADPKD patients. Furthermore, a smaller TRPP2 truncated protein in T lymphocytes of patients carrying the mutation R872X in PKD2 was also observed, suggesting that TRPP2 mutated proteins may be stably expressed. The silencing or mutation of PKD2 causes a strong reduction of ATP-evoked calcium in Jurkat cells and ADPKD2 T lymphocytes, respectively. Moreover, T lymphocytes derived from both ADPKD1 and ADPKD2 patients show increased cell proliferation, basal chemotaxis and cell aggregation compared with T lymphocytes from non-ADPKD subjects. Similarly to observations made in kidney cells, mutations in PKD1 and PKD2 dysregulate ERK, mTOR, NFkB and MIF pathways in T lymphocytes.
Conclusions: Because the alteration of ERK, mTOR, NFkB and MIF signalling found in T lymphocytes of ADPKD patients may contribute to the development of interstitial inflammation promoting cyst growth and kidney failure (ESRD), the targeting of inflammasome proteins could be an intriguing option to delay the progression of ADPKD.
Keywords: ADPKD; Calcium; ERK; NFkB; T lymphocytes; TRPP2; mTOR.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Ferreira FM, Watanabe EH, Onuchic LF. Polycystins and molecular basis of autosomal dominant polycystic kidney disease. In: Li X, editor. Polycystic kidney disease. Brisbane: Codon Publications; 2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

