Beyond the Ligand: Extracellular and Transcellular G Protein-Coupled Receptor Complexes in Physiology and Pharmacology
- PMID: 31515243
- PMCID: PMC6742926
- DOI: 10.1124/pr.119.018044
Beyond the Ligand: Extracellular and Transcellular G Protein-Coupled Receptor Complexes in Physiology and Pharmacology
Abstract
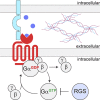
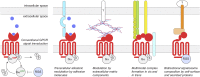
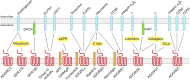
G protein-coupled receptors (GPCRs) remain one of the most successful targets of U.S. Food and Drug Administration-approved drugs. GPCR research has predominantly focused on the characterization of the intracellular interactome's contribution to GPCR function and pharmacology. However, emerging evidence uncovers a new dimension in the biology of GPCRs involving their extracellular and transcellular interactions that critically impact GPCR function and pharmacology. The seminal examples include a variety of adhesion GPCRs, such as ADGRLs/latrophilins, ADGRBs/brain angiogenesis inhibitors, ADGRG1/GPR56, ADGRG6/GPR126, ADGRE5/CD97, and ADGRC3/CELSR3. However, recent advances have indicated that class C GPCRs that contain large extracellular domains, including group III metabotropic glutamate receptors (mGluR4, mGluR6, mGluR7, mGluR8), γ-aminobutyric acid receptors, and orphans GPR158 and GPR179, can also participate in this form of transcellular regulation. In this review, we will focus on a variety of identified extracellular and transcellular GPCR-interacting partners, including teneurins, neurexins, integrins, fibronectin leucine-rich transmembranes, contactin-6, neuroligin, laminins, collagens, major prion protein, amyloid precursor protein, complement C1q-likes, stabilin-2, pikachurin, dystroglycan, complement decay-accelerating factor CD55, cluster of differentiation CD36 and CD90, extracellular leucine-rich repeat and fibronectin type III domain containing 1, and leucine-rich repeat, immunoglobulin-like domain and transmembrane domains. We provide an account on the diversity of extracellular and transcellular GPCR complexes and their contribution to key cellular and physiologic processes, including cell migration, axon guidance, cellular and synaptic adhesion, and synaptogenesis. Furthermore, we discuss models and mechanisms by which extracellular GPCR assemblies may regulate communication at cellular junctions. SIGNIFICANCE STATEMENT: G protein-coupled receptors (GPCRs) continue to be the prominent focus of pharmacological intervention for a variety of human pathologies. Although the majority of GPCR research has focused on the intracellular interactome, recent advancements have identified an extracellular dimension of GPCR modulation that alters accepted pharmacological principles of GPCRs. Herein, we describe known endogenous allosteric modulators acting on GPCRs both in cis and in trans.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
References
-
- Amalric M, Lopez S, Goudet C, Fisone G, Battaglia G, Nicoletti F, Pin JP, Acher FC. (2013) Group III and subtype 4 metabotropic glutamate receptor agonists: discovery and pathophysiological applications in Parkinson’s disease. Neuropharmacology 66:53–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

