Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea
- PMID: 31515250
- PMCID: PMC6839950
- DOI: 10.1182/blood.2019000428
Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea
Abstract
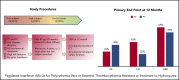
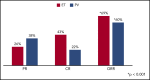
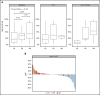
Prior studies have reported high response rates with recombinant interferon-α (rIFN-α) therapy in patients with essential thrombocythemia (ET) and polycythemia vera (PV). To further define the role of rIFN-α, we investigated the outcomes of pegylated-rIFN-α2a (PEG) therapy in ET and PV patients previously treated with hydroxyurea (HU). The Myeloproliferative Disorders Research Consortium (MPD-RC)-111 study was an investigator-initiated, international, multicenter, phase 2 trial evaluating the ability of PEG therapy to induce complete (CR) and partial (PR) hematologic responses in patients with high-risk ET or PV who were either refractory or intolerant to HU. The study included 65 patients with ET and 50 patients with PV. The overall response rates (ORRs; CR/PR) at 12 months were 69.2% (43.1% and 26.2%) in ET patients and 60% (22% and 38%) in PV patients. CR rates were higher in CALR-mutated ET patients (56.5% vs 28.0%; P = .01), compared with those in subjects lacking a CALR mutation. The median absolute reduction in JAK2V617F variant allele fraction was -6% (range, -84% to 47%) in patients achieving a CR vs +4% (range, -18% to 56%) in patients with PR or nonresponse (NR). Therapy was associated with a significant rate of adverse events (AEs); most were manageable, and PEG discontinuation related to AEs occurred in only 13.9% of subjects. We conclude that PEG is an effective therapy for patients with ET or PV who were previously refractory and/or intolerant of HU. This trial was registered at www.clinicaltrials.gov as #NCT01259856.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.Y. reports consultation and speaker honoraria for Incyte, Seattle Genetics, and Novartis. J.M. reports clinical trial research support paid to the institution from Incyte, Roche, Novartis, CTI Biopharma, Janssen, Merck, Promedior, and Celgene and membership on the clinical trial steering committee and advisory boards of Roche, CTI Biopharma, Incyte, and Celgene. J.T.P. reports clinical trial research support paid to the institution from Incyte, Abbvie, Pharmaessentia, and consulting honoraria from Agios. M.R.B. reports clinical trial research support paid to the institution from Abbvie, AI, Astellas, Forma, Incyte, Kite, and Takeda. E.W. reports serving on the advisory boards of Incyte Corporation and Gilead Sciences. A.R. reports consultation and speaker honoraria from Novartis, Amgen, Roche, Celgene, and Italfarmaco. A.M.V. reports speaker honoraria from Novartis and Celgene and fees from Novartis, CTI, Celgene for participation on advisory boards. D.R. reports consulting honoraria from Incyte. M.A. reports research grant support paid to the institution from Incyte, CTI Biopharma, Samus Therapeutics, Janssen, and Gilead. R.T.S. reports consultancy and speaker bureau for Pharmaessentia. R.R. has received consulting fees from Stemline, Celgene, Agios Pharmaceuticals, Apexx Oncology, Beyond Spring, Partner Therapeutics, and Jazz Pharmaceuticals and has received research funding from Constellation Pharmaceuticals, Incyte, and Stemline Therapeutics. R.M. reports research support from Incyte, Genetech, CTI, Promedior, and Abbvie and consulting for Novartis, Sierra Oncology, and La Jolla Pharma. R.H. reports research support from Roche. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Reducing the burden of MPN.Blood. 2019 Oct 31;134(18):1483-1484. doi: 10.1182/blood.2019002804. Blood. 2019. PMID: 31698442 No abstract available.
References
-
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. - PubMed
-
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372-375. - PubMed
-
- Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. - PubMed
-
- Alvarez-Larrán A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119(6):1363-1369. - PubMed
-
- Hernández-Boluda JC, Alvarez-Larrán A, Gómez M, et al. Clinical evaluation of the European LeukaemiaNet criteria for clinicohaematological response and resistance/intolerance to hydroxycarbamide in essential thrombocythaemia. Br J Haematol. 2011;152(1):81-88. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

