Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing
- PMID: 31515290
- PMCID: PMC7010325
- DOI: 10.1212/WNL.0000000000008243
Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing
Abstract
Objective: To use the large dataset from the Tysabri Outreach: Unified Commitment to Health (TOUCH) program to compare progressive multifocal leukoencephalopathy (PML) risk with natalizumab extended interval dosing (EID) vs standard interval dosing (SID) in patients with multiple sclerosis (MS).
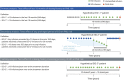
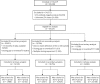
Methods: This retrospective cohort study included anti-JC virus antibody-positive patients (n = 35,521) in the TOUCH database as of June 1, 2017. The effect of EID on PML risk was evaluated with 3 planned analyses using Kaplan-Meier methods stratified by prior immunosuppressant use. Risk of PML was analyzed by Cox regression adjusted for age, sex, prior immunosuppressants, time since natalizumab initiation, and cumulative number of infusions.
Results: This study included 35,521 patients (primary analysis: 1,988 EID, 13,132 SID; secondary analysis: 3,331 EID, 15,424 SID; tertiary analysis: 815 EID, 23,168 SID). Mean average dosing intervals were 35.0 to 43.0 and 29.8 to 30.5 days for the EID and SID cohorts, respectively. Hazard ratios (95% confidence intervals) of PML risk for EID vs SID were 0.06 (0.01-0.22, p < 0.001) and 0.12 (0.05-0.29, p < 0.001) for the primary and secondary analyses, respectively. Relative risk reductions were 94% and 88% in favor of EID for the primary and secondary analyses, respectively. The tertiary analysis included no cases of PML with EID.
Conclusion: Natalizumab EID is associated with clinically and statistically significantly lower PML risk than SID.
Classification of evidence: This study provides Class III evidence that for patients with MS, natalizumab EID is associated with a lower PML risk than SID.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

Comment in
-
Dosing interval of natalizumab in MS: Do good things come to those who wait?Neurology. 2019 Oct 8;93(15):655-656. doi: 10.1212/WNL.0000000000008238. Epub 2019 Sep 12. Neurology. 2019. PMID: 31515289 No abstract available.
References
-
- Miller DH, Khan OA, Sheremata WA, et al. . A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003;348:15–23. - PubMed
-
- Polman CH, O'Connor PW, Havrdova E, et al. . A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. - PubMed
-
- Prosperini L, Sacca F, Cordioli C, et al. . Real-world effectiveness of natalizumab and fingolimod compared with self-injectable drugs in non-responders and in treatment-naive patients with multiple sclerosis. J Neurol 2017;264:284–294. - PubMed
-
- Rudick RA, Sandrock A. Natalizumab: a4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother 2004;4:571–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
