Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension
- PMID: 31515405
- PMCID: PMC6911916
- DOI: 10.1183/13993003.00378-2019
Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension
Abstract
Most published studies addressing the role of hypoxia inducible factors (HIFs) in hypoxia-induced pulmonary hypertension development employ models that may not recapitulate the clinical setting, including the use of animals with pre-existing lung/vascular defects secondary to embryonic HIF ablation or activation. Furthermore, critical questions including how and when HIF signalling contributes to hypoxia-induced pulmonary hypertension remain unanswered.Normal adult rodents in which global HIF1 or HIF2 was inhibited by inducible gene deletion or pharmacological inhibition (antisense oligonucleotides (ASO) and small molecule inhibitors) were exposed to short-term (4 days) or chronic (4-5 weeks) hypoxia. Haemodynamic studies were performed, the animals euthanised, and lungs and hearts obtained for pathological and transcriptomic analysis. Cell-type-specific HIF signals for pulmonary hypertension initiation were determined in normal pulmonary vascular cells in vitro and in mice (using cell-type-specific HIF deletion).Global Hif1a deletion in mice did not prevent hypoxia-induced pulmonary hypertension at 5 weeks. Mice with global Hif2a deletion did not survive long-term hypoxia. Partial Hif2a deletion or Hif2-ASO (but not Hif1-ASO) reduced vessel muscularisation, increases in pulmonary arterial pressures and right ventricular hypertrophy in mice exposed to 4-5 weeks of hypoxia. A small molecule HIF2 inhibitor (PT2567) significantly attenuated early events (monocyte recruitment and vascular cell proliferation) in rats exposed to 4 days of hypoxia, as well as vessel muscularisation, tenascin C accumulation and pulmonary hypertension development in rats exposed to 5 weeks of hypoxia. In vitro, HIF2 induced a distinct set of genes in normal human pulmonary vascular endothelial cells, mediating inflammation and proliferation of endothelial cells and smooth muscle cells. Endothelial Hif2a knockout prevented hypoxia-induced pulmonary hypertension in mice.Inhibition of HIF2 (but not HIF1) can provide a therapeutic approach to prevent the development of hypoxia-induced pulmonary hypertension. Future studies are needed to investigate the role of HIFs in pulmonary hypertension progression and reversal.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: C-J. Hu has nothing to disclose. Conflict of interest: J.M. Poth has nothing to disclose. Conflict of interest: H. Zhang has nothing to disclose. Conflict of interest: A. Flockton has nothing to disclose. Conflict of interest: A. Laux has nothing to disclose. Conflict of interest: S. Kumar has nothing to disclose. Conflict of interest: B. McKeon has nothing to disclose. Conflict of interest: G. Mouradian has nothing to disclose. Conflict of interest: M. Li has nothing to disclose. Conflict of interest: S. Riddle has nothing to disclose. Conflict of interest: S.C. Pugliese has nothing to disclose. Conflict of interest: R.D. Brown has nothing to disclose. Conflict of interest: E.M. Wallace has a patent “Compositions for Use in Treating Pulmonary Arterial Hypertension” pending to Peloton Therapeutics. Conflict of interest: B.B. Graham reports grants from National Institutes of Health, during the conduct of the study. Conflict of interest: M.G. Frid has nothing to disclose. Conflict of interest: K.R. Stenmark reports personal fees for advisory board or steering committee work from Pfizer, New York, Actelion (Entelligence) and Janssen Research and Development, outside the submitted work.
Figures






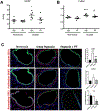
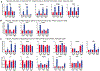
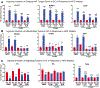

Comment in
-
Roles of HIF1 and HIF2 in pulmonary hypertension: it all depends on the context.Eur Respir J. 2019 Dec 12;54(6):1901929. doi: 10.1183/13993003.01929-2019. Print 2019 Dec. Eur Respir J. 2019. PMID: 31831673 No abstract available.
References
-
- Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2alpha. Circulation. 2016;133(24):2447–58. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
