ERS guidelines on the diagnosis and treatment of chronic cough in adults and children
- PMID: 31515408
- PMCID: PMC6942543
- DOI: 10.1183/13993003.01136-2019
ERS guidelines on the diagnosis and treatment of chronic cough in adults and children
Erratum in
-
"ERS guidelines on the diagnosis and treatment of chronic cough in adults and children." Alyn H. Morice, Eva Millqvist, Kristina Bieksiene, Surinder S. Birring, Peter Dicpinigaitis, Christian Domingo Ribas, Michele Hilton Boon, Ahmad Kantar, Kefang Lai, Lorcan McGarvey, David Rigau, Imran Satia, Jacky Smith, Woo-Jung Song, Thomy Tonia, Jan W.K. van den Berg, Mirjam J.G. van Manen and Angela Zacharasiewicz. Eur Respir J 2020; 55: 1901136.Eur Respir J. 2020 Nov 19;56(5):1951136. doi: 10.1183/13993003.51136-2019. Print 2020 Nov. Eur Respir J. 2020. PMID: 33214170 No abstract available.
Abstract
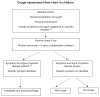
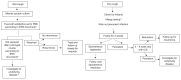
These guidelines incorporate the recent advances in chronic cough pathophysiology, diagnosis and treatment. The concept of cough hypersensitivity has allowed an umbrella term that explains the exquisite sensitivity of patients to external stimuli such a cold air, perfumes, smoke and bleach. Thus, adults with chronic cough now have a firm physical explanation for their symptoms based on vagal afferent hypersensitivity. Different treatable traits exist with cough variant asthma (CVA)/eosinophilic bronchitis responding to anti-inflammatory treatment and non-acid reflux being treated with promotility agents rather the anti-acid drugs. An alternative antitussive strategy is to reduce hypersensitivity by neuromodulation. Low-dose morphine is highly effective in a subset of patients with cough resistant to other treatments. Gabapentin and pregabalin are also advocated, but in clinical experience they are limited by adverse events. Perhaps the most promising future developments in pharmacotherapy are drugs which tackle neuronal hypersensitivity by blocking excitability of afferent nerves by inhibiting targets such as the ATP receptor (P2X3). Finally, cough suppression therapy when performed by competent practitioners can be highly effective. Children are not small adults and a pursuit of an underlying cause for cough is advocated. Thus, in toddlers, inhalation of a foreign body is common. Persistent bacterial bronchitis is a common and previously unrecognised cause of wet cough in children. Antibiotics (drug, dose and duration need to be determined) can be curative. A paediatric-specific algorithm should be used.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: A.H. Morice reports grants, personal fees, non-financial support and other (advisory board participation) from Merck Sharp & Dohme Corp., Bayer AG Research & Development, Bayer US, Sanofi and Phillips Respironics, personal fees, non-financial support and other (advisory board participation) from Bellus Health, personal fees and non-financial support from AstraZeneca, Chiesi Ltd and Boehringer Ingelheim, grants, personal fees and non-financial support from GlaxoSmithKline and Respivant Sciences, Inc., grants, personal fees and other (advisory board participation) from NeRRe Therapeutics, grants from Menlo Therapeutics, during the conduct of the study. Conflict of interest: E. Millqvist filed an international patent application (PCT application) for the use of capsaicin as a cough-reducing product on January 3, 2014. There is a pending patent application in the USA, Canada and the EU. In Australia a patent was issued on August 17 2017. However, this treatment method is not described, recommended or emphasised in any way in the guidelines. Conflict of interest: K. Bieksiene has nothing to disclose. Conflict of interest: S.S. Birring reports grants from Merck, personal fees for advisory board work from Merck, Bayer, GSK, Menlo and Sanofi, travel expenses reimbursement from Boehringer Ingleheim, outside the submitted work. Conflict of interest: P. Dicpinigaitis has nothing to disclose. Conflict of interest: C. Domingo Ribas reports personal fees for lectures and advisory board participation from MSD, AstraZeneca, ALK and Sanofi-Aventis, personal fees for lectures, advisory board participation and non-financial support (study collaboration) from Novartis and Teva, personal fees for meeting attendance from Allergy Therapeutics, Immunotek, Esteve and Menarini, personal fees from Chiesi, personal fees for lectures from Ferrer, non-financial support (study collaboration) from GSK, outside the submitted work. Conflict of interest: M. Hilton Boon reports grants from Medical Research Council (UK) (MC_UU_12017/15), and from Scottish Government Chief Scientist Office (SPHSU15), during the conduct of the study. Conflict of interest: A. Kantar is advisor for study design of an unlicensed product (Merck Sharp & Dohme, USA); advisor for study design of an over-the-counter product (Sanofi, Germany) and (Infirst, UK). No financial or intellectual conflicts of interest regarding the content of this manuscript. Conflict of interest: K. Lai reports grants and personal fees from AstraZeneca, GlaxoSmithKline, Merck, Daiyichi Sankyo and Novartis, outside the submitted work. Conflict of interest: L. McGarvey reports personal fees from Merck & Co., Inc. and AstraZeneca, grants, personal fees and non-financial support from Chiesi, personal fees and non-financial support from Boehringer Ingelheim, grants and non-financial support from GlaxoSmithKline, grants and personal fees from Almirall, grants from NC3R, during the conduct of the study; grants from European Union Interreg VA Health & Life Science Programme, outside the submitted work. Conflict of interest: D. Rigau acts as methodologist for the ERS. Conflict of interest: I. Satia is currently supported by ERS Marie Curie Respire 3 Global Fellowship programme (713406), and reports grants from BMA James Trust Award, grants from North West Lung Centre Charity Grant, personal fees for lectures from GSK and AstraZeneca, travel Awards for attending conferences from GSK and Chiesi, outside the submitted work. Conflict of interest: J. Smith reports grants and personal fees for consultancy and advisory board work from GlaxoSmithKline, grants and personal fees for consultancy from NeRRe Pharmaceuticals, Menlo, Bayer, Axalbion and Merck, personal fees for consultancy from Boehringer Ingelheim, Genentech, Neomed, Chiesi and Bellus, non-financial support (provision of cough monitoring equipment) from Vitalograph, grants and personal fees from Afferent, personal fees for consultancy and non-financial support (scientific collaboration support in kind) from AstraZeneca, outside the submitted work; and has a patented method for generating output data licensed. Conflict of interest: W-J. Song has nothing to disclose. Conflict of interest: T. Tonia acts as ERS methodologist. Conflict of interest: J.W.K. van den Berg reports personal fees for advisory board work from MSD, grants from Bayer, outside the submitted work. Conflict of interest: M.J.G. van Manen has nothing to disclose. Conflict of interest: A. Zacharasiewicz reports payment for consultancy work for Vertex, Novartis, Abbvie and Loewenstein, travel sponsorship from Mylan, Chiesi and Teva, support for research from Abbvie.
References
-
- Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, Jo EJ, Kim MH, Plevkova J, Park HW, Cho SH, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479–1481. - PubMed
-
- Chamberlain SA, Garrod R, Douiri A, Masefield S, Powell P, Bucher C, Pandyan A, Morice AH, Birring SS. The impact of chronic cough: a cross-sectional European survey. Lung. 2015;193(3):401–408. - PubMed
-
- Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B, Smith JA, Parker SM, Chung KF, Lai K, Pavord ID, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. The European respiratory journal. 2014;44(5):1149–1155. - PubMed
-
- Millqvist E. The airway sensory hyperreactivity syndrome. PulmPharmacolTher. 2011;24(3):263–266. - PubMed
-
- Morice AH, Millqvist E, Belvisi MG, Bieksiene K, Birring SS, Chung KF, Dal Negro RW, Dicpinigaitis P, Kantar A, McGarvey LP, Pacheco A, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. The European respiratory journal. 2014;44(5):1132–1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical