Earlier Degraded Tapetum1 (EDT1) Encodes an ATP-Citrate Lyase Required for Tapetum Programmed Cell Death
- PMID: 31515447
- PMCID: PMC6836821
- DOI: 10.1104/pp.19.00202
Earlier Degraded Tapetum1 (EDT1) Encodes an ATP-Citrate Lyase Required for Tapetum Programmed Cell Death
Abstract
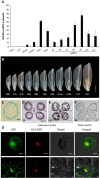
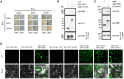
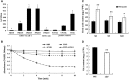
In flowering plants, the tapetum cells in anthers undergo programmed cell death (PCD) at the late meiotic stage, providing nutrients for further development of microspores, including the formation of the pollen wall. However, the molecular basis of tapetum PCD remains elusive. Here we report a tapetum PCD-related mutant in rice (Oryza sativa), earlier degraded tapetum 1 (edt1), that shows complete pollen abortion associated with earlier-than-programmed tapetum cell death. EDT1 encodes a subunit of ATP-citrate lyase (ACL), and is specifically expressed in the tapetum of anthers. EDT1 localized in both the nucleus and the cytoplasm as observed in rice protoplast transient assays. We demonstrated that the A and B subunits of ACL interacted with each other and might function as a heteromultimer in the cytoplasm. EDT1 catalyzes the critical steps in cytosolic acetyl-CoA synthesis. Our data indicated a decrease in ATP level, energy charge, and fatty acid content in mutant edt1 anthers. In addition, the genes encoding secretory proteases or lipid transporters, and the transcription factors known to regulate PCD, were downregulated. Our results demonstrate that the timing of tapetum PCD must be tightly regulated for successful pollen development, and that EDT1 is involved in the tapetum PCD process. This study furthers our understanding of the molecular basis of pollen fertility and fecundity in rice and may also be relevant to other flowering plants.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures






References
-
- Ahlers F, Bubert H, Steuernagel S, Wiermann R (2000) The nature of oxygen in sporopollenin from the pollen of Typha angustifolia L. Z Naturforsch C 55: 129–136 - PubMed
-
- Ahlers F, Lambert J, Wiermann R (2003) Acetylation and silylation of piperidine solubilized sporopollenin from pollen of Typha angustifolia L. Z Naturforsch C 58: 807–811 - PubMed
-
- Balcke GU, Bennewitz S, Bergau N, Athmer B, Henning A, Majovsky P, Jiménez-Gómez JM, Hoehenwarter W, Tissier A (2017) Multi-omics of tomato glandular trichomes reveals distinct features of central carbon metabolism supporting high productivity of specialized metabolites. Plant Cell 29: 960–983 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

