Response to Anti-EGFR Therapy in Patients with BRAF non-V600-Mutant Metastatic Colorectal Cancer
- PMID: 31515458
- PMCID: PMC6891165
- DOI: 10.1158/1078-0432.CCR-19-2004
Response to Anti-EGFR Therapy in Patients with BRAF non-V600-Mutant Metastatic Colorectal Cancer
Abstract
Purpose: While mutations in BRAF in metastatic colorectal cancer (mCRC) most commonly occur at the V600 amino acid, with the advent of next-generation sequencing, non-V600 BRAF mutations are increasingly identified in clinical practice. It is unclear whether these mutants, like BRAF V600E, confer resistance to anti-EGFR therapy.
Experimental design: We conducted a multicenter pooled analysis of consecutive patients with non-V600 BRAF-mutated mCRCs identified between 2010 and 2017. Non-V600 BRAF mutations were divided into functional classes based on signaling mechanism and kinase activity: activating and RAS-independent (class 2) or kinase-impaired and RAS-dependent (class 3).
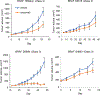
Results: Forty patients with oncogenic non-V600 BRAF-mutant mCRC received anti-EGFR antibody treatment [n = 12 (30%) class 2 and n = 28 (70%) class 3]. No significant differences in clinical characteristics were observed by mutation class. In contrast, while only 1 of 12 patients with class 2 BRAF mCRC responded, 14 of 28 patients with class 3 BRAF responded to anti-EGFR therapy (response rate, 8% and 50%, respectively, P = 0.02). Specifically, in first- or second-line, 1 of 6 (17%) patients with class 2 and 7 of 9 (78%) patients with class 3 BRAF mutants responded (P = 0.04). In third- or later-line, none of 6 patients with class 2 and 7 of 19 (37%) patients with class 3 BRAF mutants responded (P = 0.14).
Conclusions: Response to EGFR antibody treatment in mCRCs with class 2 BRAF mutants is rare, while a large portion of CRCs with class 3 BRAF mutants respond. Patients with colorectal cancer with class 3 BRAF mutations should be considered for anti-EGFR antibody treatment.See related commentary by Fontana and Valeri, p. 6896.
©2019 American Association for Cancer Research.
Figures
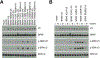
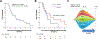
Comment in
-
Class(y) Dissection of BRAF Heterogeneity: Beyond Non-V600.Clin Cancer Res. 2019 Dec 1;25(23):6896-6898. doi: 10.1158/1078-0432.CCR-19-2732. Epub 2019 Oct 4. Clin Cancer Res. 2019. PMID: 31585936
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

