A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein
- PMID: 31515478
- PMCID: PMC6742648
- DOI: 10.1038/s41467-019-12137-1
A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein
Abstract
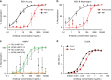
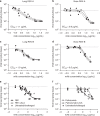
Respiratory syncytial virus (RSV) infection is the leading cause of hospitalization and infant mortality under six months of age worldwide; therefore, the prevention of RSV infection in all infants represents a significant unmet medical need. Here we report the isolation of a potent and broadly neutralizing RSV monoclonal antibody derived from a human memory B-cell. This antibody, RB1, is equipotent on RSV A and B subtypes, potently neutralizes a diverse panel of clinical isolates in vitro and demonstrates in vivo protection. It binds to a highly conserved epitope in antigenic site IV of the RSV fusion glycoprotein. RB1 is the parental antibody to MK-1654 which is currently in clinical development for the prevention of RSV infection in infants.
Conflict of interest statement
A.T., Z.C., K.C, HP.S, C.C., A.F., L.Z., S. P., P.C.,R.S., S.T., M.C, B.L., M.E., J.R., S.S., J.G., D.W., Z.W., G.H., D.D. and K.V. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may hold stock in Merck & Co., Inc., Kenilworth, NJ, USA. D.C. and D.G. are former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may hold stock in Merck & Co., Inc., Kenilworth, NJ, USA. D.C. is an employee of Sanofi Pasteur. D.G. is an employee of Janssen Research and Development.
Figures
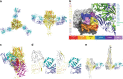
References
-
- Leader, S. & Kohlhase, K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J. Pediatr. 143, S127–S132 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

