Differentiation but not ALS mutations in FUS rewires motor neuron metabolism
- PMID: 31515480
- PMCID: PMC6742665
- DOI: 10.1038/s41467-019-12099-4
Differentiation but not ALS mutations in FUS rewires motor neuron metabolism
Abstract
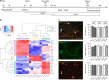
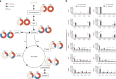
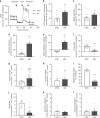
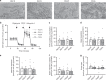
Energy metabolism has been repeatedly linked to amyotrophic lateral sclerosis (ALS). Yet, motor neuron (MN) metabolism remains poorly studied and it is unknown if ALS MNs differ metabolically from healthy MNs. To address this question, we first performed a metabolic characterization of induced pluripotent stem cells (iPSCs) versus iPSC-derived MNs and subsequently compared MNs from ALS patients carrying FUS mutations to their CRISPR/Cas9-corrected counterparts. We discovered that human iPSCs undergo a lactate oxidation-fuelled prooxidative metabolic switch when they differentiate into functional MNs. Simultaneously, they rewire metabolic routes to import pyruvate into the TCA cycle in an energy substrate specific way. By comparing patient-derived MNs and their isogenic controls, we show that ALS-causing mutations in FUS did not affect glycolytic or mitochondrial energy metabolism of human MNs in vitro. These data show that metabolic dysfunction is not the underlying cause of the ALS-related phenotypes previously observed in these MNs.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Aberrant axon branching via Fos-B dysregulation in FUS-ALS motor neurons.EBioMedicine. 2019 Jul;45:362-378. doi: 10.1016/j.ebiom.2019.06.013. Epub 2019 Jun 29. EBioMedicine. 2019. PMID: 31262712 Free PMC article.
-
4-Aminopyridine Induced Activity Rescues Hypoexcitable Motor Neurons from Amyotrophic Lateral Sclerosis Patient-Derived Induced Pluripotent Stem Cells.Stem Cells. 2016 Jun;34(6):1563-75. doi: 10.1002/stem.2354. Epub 2016 Mar 28. Stem Cells. 2016. PMID: 26946488
-
Altered calcium dynamics and glutamate receptor properties in iPSC-derived motor neurons from ALS patients with C9orf72, FUS, SOD1 or TDP43 mutations.Hum Mol Genet. 2019 Sep 1;28(17):2835-2850. doi: 10.1093/hmg/ddz107. Hum Mol Genet. 2019. PMID: 31108504
-
[The FUS protein: Physiological functions and a role in amyotrophic lateral sclerosis].Mol Biol (Mosk). 2017 May-Jun;51(3):387-399. doi: 10.7868/S0026898417020094. Mol Biol (Mosk). 2017. PMID: 28707655 Review. Russian.
-
[ALS disease modeling and drug screening using patient-specific iPS cells].Rinsho Shinkeigaku. 2013;53(11):1020-2. doi: 10.5692/clinicalneurol.53.1020. Rinsho Shinkeigaku. 2013. PMID: 24291866 Review. Japanese.
Cited by
-
The multifaceted role of kinases in amyotrophic lateral sclerosis: genetic, pathological and therapeutic implications.Brain. 2020 Jun 1;143(6):1651-1673. doi: 10.1093/brain/awaa022. Brain. 2020. PMID: 32206784 Free PMC article. Review.
-
Aberrant CHCHD2-associated mitochondriopathy in Kii ALS/PDC astrocytes.Acta Neuropathol. 2024 May 15;147(1):84. doi: 10.1007/s00401-024-02734-w. Acta Neuropathol. 2024. PMID: 38750212
-
Mitochondrial dysfunction of induced pluripotent stem cells-based neurodegenerative disease modeling and therapeutic strategy.Front Cell Dev Biol. 2022 Nov 21;10:1030390. doi: 10.3389/fcell.2022.1030390. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36478742 Free PMC article. Review.
-
Investigation of mitochondrial phenotypes in motor neurons derived by direct conversion of fibroblasts from familial ALS subjects.bioRxiv [Preprint]. 2025 Feb 17:2025.02.13.637962. doi: 10.1101/2025.02.13.637962. bioRxiv. 2025. PMID: 40027671 Free PMC article. Preprint.
-
Unveiling amyotrophic lateral sclerosis complexity: insights from proteomics, metabolomics and microbiomics.Brain Commun. 2025 Mar 19;7(2):fcaf114. doi: 10.1093/braincomms/fcaf114. eCollection 2025. Brain Commun. 2025. PMID: 40161216 Free PMC article. Review.
References
-
- Borthwick Gillian M., Johnson Margaret A., Ince Paul G., Shaw Pamela J., Turnbull Douglass M. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: Implications for the role of mitochondria in neuronal cell death. Annals of Neurology. 1999;46(5):787–790. doi: 10.1002/1531-8249(199911)46:5<787::AID-ANA17>3.0.CO;2-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

