Peripheral changes in immune cell populations and soluble mediators after anti-PD-1 therapy in non-small cell lung cancer and renal cell carcinoma patients
- PMID: 31515670
- PMCID: PMC11028077
- DOI: 10.1007/s00262-019-02391-z
Peripheral changes in immune cell populations and soluble mediators after anti-PD-1 therapy in non-small cell lung cancer and renal cell carcinoma patients
Abstract
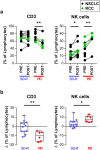
Patients with non-small cell lung cancer (NSCLC) and renal cell carcinoma (RCC) have shown benefit from anti-PD-1 therapies. However, not all patients experience tumor shrinkage, durable responses or prolonged survival, demonstrating the need to find response markers. In blood samples from NSCLC and RCC patients obtained before and after anti-PD-1 treatment, we studied leukocytes by complete blood cell count, lymphocyte subsets using flow cytometry and plasma concentration of nine soluble mediators, in order to find predictive biomarkers of response and to study changes produced after anti-PD-1 therapy. In baseline samples, discriminant analysis revealed a combination of four variables that helped differentiate stable disease-response (SD-R) from progressive disease (PD) patients: augmented frequency of central memory CD4+ T cells and leukocyte count was associated with response while increased percentage of PD-L1+ natural killer cells and naïve CD4+ T cells was associated with lack of response. After therapy, differential changes between responders and non-responders were found in leukocytes, T cells and TIM-3+ T cells. Patients with progressive disease showed an increase in the frequency of TIM-3 expressing CD4+ and CD8+ T cells, whereas SD-R patients showed a decrease in these subsets. Our findings indicate that a combination of immune variables from peripheral blood (PB) could be useful to distinguish response groups in NSCLC and RCC patients treated with anti-PD-1 therapy. Frequency of TIM-3+ T cells showed differential changes after treatment in PD vs SD-R patients, suggesting that it may be an interesting marker for monitoring progression during therapy.
Keywords: Anti-PD-1 therapy; NSCLC; Nivolumab; Pembrolizumab; Renal cell carcinoma; TIM-3.
Conflict of interest statement
Claudio Martín has served as speaker and advisory board member for Bristol Myers Squibb and Merck Sharp and Dohme. Carmen Pupareli has served as speaker and advisor board member for Merck Sharp and Dohme and as speaker for Bristol Myers Squibb. The authors declare that there is no other conflict of interest.
Figures




References
-
- Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

