A multicentre, open-label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors
- PMID: 31515851
- PMCID: PMC6900083
- DOI: 10.1111/hae.13848
A multicentre, open-label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors
Abstract
Introduction: Emicizumab is a recombinant humanized bispecific monoclonal antibody mimicking the cofactor function of activated factor VIII.
Aim: In this multicentre, open-label study (HOHOEMI), we evaluated the efficacy, safety and pharmacokinetics of emicizumab in Japanese paediatric patients aged <12 years with severe haemophilia A without factor VIII (FVIII) inhibitors.
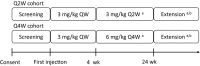
Methods: Emicizumab was administered subcutaneously, with four loading doses of 3 mg/kg every week followed by maintenance doses of 3 mg/kg every 2 weeks (Q2W) or 6 mg/kg every 4 weeks (Q4W) in 6 and 7 patients, respectively.
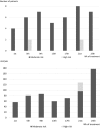
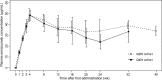
Results: All patients completed at least 24 weeks of treatment. Baseline ages ranged from 4 months to 10 years, and all patients had been treated with FVIII prophylaxis prior to enrolment except a 4-month-old patient untreated with FVIII previously. In the respective Q2W and Q4W cohorts, 2/6 and 5/7 patients experienced no treated bleeding events, and annualized bleeding rates for treated bleeding events were 1.3 (95% confidence interval [CI], 0.6-2.9) and 0.7 (95% CI, 0.2-2.6). All caregivers preferred emicizumab to the patient's previous treatment. Only one related adverse event (injection site reaction) was observed. There were no thromboembolic events or thrombotic microangiopathy. Individual trough plasma concentrations of emicizumab were within the variability observed in preceding adult/adolescent studies. All patients tested negative for anti-emicizumab antibodies.
Conclusions: Emicizumab administered Q2W or Q4W was efficacious and safe in paediatric patients with severe haemophilia A without inhibitors. This study was registered at http://www.clinicaltrials.jp (JapicCTI-173710).
Keywords: bispecific antibody; emicizumab; haemophilia A; non-inhibitor; paediatrics; prophylaxis.
© 2019 The Authors. Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
This study was sponsored by Chugai Pharmaceutical Co., Ltd. M. Shima received research funding from Chugai Pharmaceutical Co., Ltd., F. Hoffmann‐La Roche Ltd., Bioverativ Inc, Shire Plc, CSL Behring, KM Biologics Co., Ltd. and Novo Nordisk A/S; consulting fee from Chugai Pharmaceutical Co., Ltd.; payment for lectures on speaker's bureau from Chugai Pharmaceutical Co., Ltd., Bioverativ Inc, Bayer AG and Sysmex corporation; is listed as an entity's board of directors or advisory committee member for Chugai Pharmaceutical Co., Ltd., F. Hoffmann‐La Roche Ltd., BioMarin Pharmaceutical Inc, Bayer AG and Sanofi SA; and is an inventor of patents related to anti‐FIXa/FX bispecific antibodies. K. Nogami received research funding from Chugai Pharmaceutical Co., Ltd., F. Hoffmann‐La Roche Ltd., Shire Plc, Bioverativ Inc, Novo Nordisk A/S and Bayer AG; consulting fee from Chugai Pharmaceutical Co., Ltd.; payment for lectures on speaker's bureau from Chugai Pharmaceutical Co., Ltd., Shire Plc, Bioverativ Inc, Novo Nordisk A/S and Bayer AG; is listed as an entity's board of directors or advisory committee member for Chugai Pharmaceutical Co., Ltd. and F. Hoffmann‐La Roche Ltd.; and is an inventor of patents related to anti‐FIXa/FX bispecific antibodies. S. Nagami is an employee of Chugai Pharmaceutical Co., Ltd. and holds stock in Chugai Pharmaceutical Co., Ltd. S. Yoshida is an employee of Chugai Pharmaceutical Co., Ltd. K. Yoneyama is an employee of Chugai Pharmaceutical Co., Ltd. and is an inventor of patents related to anti‐FIXa/FX bispecific antibodies. A. Ishiguro received research funding from Chugai Pharmaceutical Co., Ltd., F. Hoffmann‐La Roche Ltd., Novo Nordisk A/S, Pfizer Inc, KM Biologics Co., Ltd. and Teijin Pharma Ltd.; consulting fee from Novo Nordisk A/S; and payment for lectures on speaker's bureau from Chugai Pharmaceutical Co., Ltd., Novo Nordisk A/S and Eisai Co., Ltd. T. Suzuki received research funding from Chugai Pharmaceutical Co., Ltd., F. Hoffmann‐La Roche Ltd., Novo Nordisk A/S, BioMarin Pharmaceutical Inc, Shire Plc, Octapharma AG, Bayer AG, Pfizer Inc and Bioverativ Inc; and payment for lectures on speaker's bureau from Chugai Pharmaceutical Co., Ltd., Novo Nordisk A/S, Shire Plc, Bayer AG, Pfizer Inc, Bioverativ Inc, CSL Behring, KM Biologics Co., Ltd., Nihon Pharmaceutical Co., Ltd., Sekisui Medical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., LSI Medience Corporation
Figures

References
-
- Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1‐e47. - PubMed
-
- Fischer K, Lewandowski D, Marijke van den Berg H, Janssen MP. Validity of assessing inhibitor development in haemophilia PUPs using registry data: the EUHASS project. Haemophilia. 2012;18:e241‐e246. - PubMed
-
- Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374:2054‐2064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

