New Direct-Acting Antivirals for the Treatment of Patients With Hepatitis C Virus Infection: A Systematic Review of Randomized Controlled Trials
- PMID: 31516269
- PMCID: PMC6728536
- DOI: 10.1016/j.jceh.2018.07.004
New Direct-Acting Antivirals for the Treatment of Patients With Hepatitis C Virus Infection: A Systematic Review of Randomized Controlled Trials
Abstract
Background: New direct-acting antiviral agents (DAAs) approved for the treatment of patients infected by Hepatitis C virus (HCV) are well tolerated and increase sustained virological response (SVR) rate. We summarize current evidence on the efficacy and safety from comparative randomized controlled trials (RCTs) of DAAs.
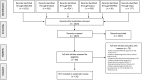
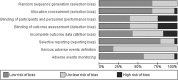
Methods: We systematically searched MEDLINE, Embase, Scopus, CENTRAL, and Lilacs as well as a list of reference literature. We included RCTs comparing DAAs with placebo or active control and reporting response rates and adverse events according to antiviral regimens. Risk ratios (RRs) were pooled as appropriate. We assessed the risk of bias of included studies and graded the quality of evidence according to the GRADE method.
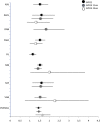
Results: We included 28 RCTs, enrolling more than 7000 patients. The quality of evidence was generally low. Twelve-week treatment with DAAs in naïve patients significantly increased SVR12 and SVR24 compared with placebo (RR 1.4, 95% CI 1.3-1.6; RR 1.5, 95% CI 1.4-1.6, respectively). This means that for every 1000 patients, 240 or 260 more patients experienced SVR12 or SVR24 if treated with any DAAs. We could not find RCTs assessing progression of liver disease or development of hepatocellular carcinoma. DAAs were not associated with higher incidence of serious adverse events or discontinuation due to adverse events.
Conclusions: This systematic review confirms that new DAAs are more effective in inducing SVR than placebo. Outside clinical trials, in real word, HCV cure with DAA regimens occurs in less than 90% of patients, so further comparative evaluations are needed to establish their long-term effects.
Keywords: AE, adverse event; CI, confidence interval; DAA, direct-acting antiviral agent; HCC, hepatocellular carcinoma; HCV, Hepatitis C virus; NNPIs, nonnucleoside polymerase inhibitors; NPIs, nucleoside polymerase inhibitors; PEG-IFN, pegylated interferon; PrIs, protease inhibitors; RAVs, resistance-associated variants; RBV, Ribavirin; RCT, randomized controlled trial; RR, risk ratio; SAEs, serious adverse events; SE, standard error; SVR, sustained virological response; hepatitis C; liver; meta-analysis; outcome research; systematic review.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources