Percutaneous closure of patent ductus arteriosus with the Nit-Occlud® patent ductus arteriosus device in 268 consecutive cases
- PMID: 31516276
- PMCID: PMC6716305
- DOI: 10.4103/apc.APC_151_18
Percutaneous closure of patent ductus arteriosus with the Nit-Occlud® patent ductus arteriosus device in 268 consecutive cases
Abstract
Background: The pfm Nit-Occlud® patent ductus arteriosus (PDA) device is well established for interventional closure of PDA. However, there are still limited data concerning its efficacy and follow-up in larger patient groups.
Aims: This study aimed to evaluate the safety and efficacy of the Nit-Occlud® PDA device, implanted both through transpulmonary and transaortic approach, in a large cohort.
Methods: From July 2008 to December 2015, 268 consecutive patients were admitted for transcatheter closure of a PDA and were treated with the Nit-Occlud® coil. Clinical, echocardiographic, and angiographic data were evaluated.
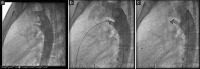
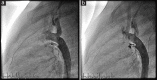
Results: The median age was 5.2 years (range, 5 months to 62 years), and the median weight was 19.3 kg (range: 5.5-97 kg). Ten (3.7%) patients had weight <10 kg. The most common ductus types treated were Krichenko Type E and A (44.0% and 33.2%, respectively). Twelve (4.5%) patients were treated for residual shunting after surgical PDA closure. The median diameter at the narrowest point was 1.5 mm (range: 0.4-4 mm), the median size of the ampulla was 5 mm (range: 1-15 mm), and the median length was 9 mm (range: 2-25 mm). Device implantation could be successfully achieved in all cases. Closure rates documented immediately after the procedure, at 3-10 days, 1 month, and 6 months after intervention were 62%, 95.1%, 97.8%, and 98.5%, respectively. With the exception of one minor thromboembolic event, there were no procedure-related complications.
Conclusion: Closure of PDA with various anatomic variations and sizes can be performed effectively and safely using the Nit-Occlud® coil.
Keywords: Interventional closure; interventional devices; patent ductus arteriosus.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Porstmann W, Wierny L, Warnke H. Closure of persistent ductus arteriosus without thoracotomy. Ger Med Mon. 1967;12:259–61. - PubMed
-
- Baruteau AE, Hascoët S, Baruteau J, Boudjemline Y, Lambert V, Angel CY, et al. Transcatheter closure of patent ductus arteriosus: Past, present and future. Arch Cardiovasc Dis. 2014;107:122–32. - PubMed
-
- Pass RH, Hijazi Z, Hsu DT, Lewis V, Hellenbrand WE. Multicenter USA Amplatzer patent ductus arteriosus occlusion device trial: Initial and one-year results. J Am Coll Cardiol. 2004;44:513–9. - PubMed
-
- Moore JW, Greene J, Palomares S, Javois A, Owada CY, Cheatham JP, et al. Results of the combined U.S. Multicenter pivotal study and the continuing access study of the nit-occlud PDA device for percutaneous closure of patent ductus arteriosus. JACC Cardiovasc Interv. 2014;7:1430–6. - PubMed
-
- Brunetti MA, Ringel R, Owada C, Coulson J, Jennings JM, Hoyer MH, et al. Percutaneous closure of patent ductus arteriosus: A multiinstitutional registry comparing multiple devices. Catheter Cardiovasc Interv. 2010;76:696–702. - PubMed

