Dataset of asymmetric intramolecular [4+3] cycloaddition reactions catalyzed by NHC-gold(I) complexes
- PMID: 31516945
- PMCID: PMC6727039
- DOI: 10.1016/j.dib.2019.104409
Dataset of asymmetric intramolecular [4+3] cycloaddition reactions catalyzed by NHC-gold(I) complexes
Abstract
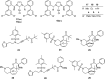
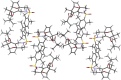
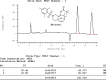
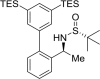
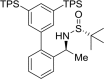
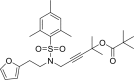
The shared data is the unpublished portion of the experimental section for the article with the title "NHC-Au(I) catalyzed enantioselective intramolecular [4 + 3] cycloaddition of furan propargyl esters".[1] The preparation of the intermediates for chiral NHC-gold(I) complexes and the furan propargyl ester substrates are included in this article. The 1H NMR and 13C NMR spectra of the gold complexes 17a-19c and the X-ray crystal data of 17a, 18a and cycloaddition product 24 are also provided in this article or in Mendeley Data. Finally, the chiral HPLC spectra used to determine enantiomeric excess and Cartesian coordinates of the optimized structure of 25 and 26 calculated by DFT calculation are also presented in the article.
Keywords: Cycloaddition; DFT calculation; Enantioselective; NHC-gold(I) complexes.
Figures















References
-
- Ma R., Yang J., Kelley S., Gung B.W. NHC–Au(I)catalyzed enantioselective intramolecular [4+3] cycloaddition of furan propargyl esters. J. Organomet. Chem. 2019;898
-
- Gung B.W., Holmes M.R., Jones C.A., Ma R., Barnes C.L. Structure–enantioselectivity correlation in NHC–Au(I)catalysis for 1,6-enynecyclizations. Tetrahedron Lett. 2016;57:3912–3915.
-
- Campolo D., Arif T., Borie C., Mouysset D., Vanthuyne N., Naubron J.V., Bertrand M.P., Nechab M. Double transfer of chirality in organocopper-mediated bis(alkylating)cycloisomerization of enediynes. Angew. Chem. Int. Ed. 2014;53:3227–3231. - PubMed
-
- Li G., Zhang G., Zhang L. Au-catalyzed synthesis of (1Z,3E)-2-pivaloxy-1,3-dienes from propargylic pivalates. J. Am. Chem. Soc. 2008;130:3740–3741. - PubMed
LinkOut - more resources
Full Text Sources

