Prospective Randomized Controlled Trial on the Efficacy of Continuous Positive Airway Pressure and Adaptive Servo-Ventilation in the Treatment of Chronic Complex Insomnia
- PMID: 31517263
- PMCID: PMC6734001
- DOI: 10.1016/j.eclinm.2019.06.011
Prospective Randomized Controlled Trial on the Efficacy of Continuous Positive Airway Pressure and Adaptive Servo-Ventilation in the Treatment of Chronic Complex Insomnia
Abstract
Background: Complex insomnia, the comorbidity of chronic insomnia and obstructive sleep apnea (OSA), is a common sleep disorder, but the OSA component, whether presenting overtly or covertly, often goes unsuspected and undiagnosed due to a low index of suspicion. Among complex insomniacs, preliminary evidence demonstrates standard CPAP decreases insomnia severity. However, CPAP causes expiratory pressure intolerance or iatrogenic central apneas that may diminish its use. An advanced PAP mode-adaptive servo-ventilation (ASV)-may alleviate CPAP side-effects and yield superior outcomes.
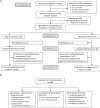
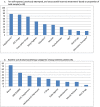
Methods: In a single-site protocol investigating covert complex insomnia (ClinicalTrials.gov identifier: NCT02365064), a low index of suspicion for this comorbidity was confirmed by exclusion of 455 of 660 eligible patients who presented during the study period with overt OSA signs and symptoms. Ultimately, stringent inclusion/exclusion criteria to test efficacy yielded 40 adult, covert complex insomnia patients [average Insomnia Severity Index (ISI) moderate-severe 19.30 (95% CI 18.42-20.17)] who reported no definitive OSA symptoms or risks and who failed behavioral or drug therapy for an average of one decade. All 40 were diagnosed with OSA and randomized (using block randomization) to a single-blind, prospective protocol, comparing CPAP (n = 21) and ASV (n = 19). Three successive PAP titrations fine-tuned pressure settings, facilitated greater PAP use, and collected objective sleep and breathing data. Patients received 14 weeks of treatment including intensive biweekly coaching and follow-up to foster regular PAP use in order to accurately measure efficaciousness. Primary outcomes measured insomnia severity and sleep quality. Secondary outcomes measured daytime impact: OSA-induced impairment, fatigue severity, insomnia impairment, and quality of life. Performance on these seven variables was assessed using repeated measures ANCOVA to account for the multiple biweekly time points.
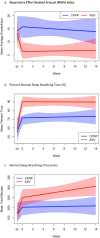
Findings: At intake, OSA diagnosis and OSA as a cause for insomnia were denied by all 40 patients, yet PAP significantly decreased insomnia severity scores (p = 0.021 in the primary ANCOVA analysis). To quantify effect sizes, mean intake vs endpoint analysis was conducted with ASV yielding nearly twice the effects of CPAP [- 13.2 (10.7-15.7), Hedges' g = 2.50 vs - 9.3 (6.3-12.3), g = 1.39], and between mode effect size was in the medium-large range 0.65. Clinically, ASV led to remission (ISI < 8) in 68% of cases compared to 24% on CPAP [Fisher's exact p = 0.010]. Two sleep quality measures in the ANCOVA analysis again demonstrated superior significant effects for ASV compared to CPAP (both p < 0.03), and pre- and post-analysis demonstrated substantial effects for both scales [ASV (g = 1.42; g = 1.81) over CPAP (g = 1.04; g = 0.75)] with medium size effects between modes (0.54, 0.51). Measures of impairment, residual objective sleep breathing events, and normalized breathing periods consistently demonstrated larger beneficial effects for ASV over CPAP.
Interpretation: PAP therapy was highly efficacious in decreasing insomnia severity in chronic insomnia patients with previously undiagnosed co-morbid OSA. ASV proved superior to CPAP in this first efficacy trial to compare advanced to traditional PAP modes in complex insomnia. Future research must determine the following: pathophysiological mechanisms to explain how OSA causes chronic insomnia; general population prevalence of this comorbidity; and, cost-effectiveness of ASV therapy in complex insomnia. Last, efforts to raise awareness of complex insomnia are urgently needed as patients and providers appear to disregard both overt and covert signs and symptoms of OSA in the assessment of chronic insomnia.
Keywords: Adaptive servo-ventilation; Apnea; Complex insomnia; Continuous positive airway pressure; Efficacy trial; Hypopnea; Insomnia; Obstructive sleep apnea; Respiratory effort-related arousals; Sleep fragmentation; Sleep quality; Upper airway resistance syndrome.
Conflict of interest statement
Authors NDM, VAU, JK, and RMS report grants from ResMed Sciences during the conduct of the study. Additionally, author JK is the CEO of the commercial sleep center, Maimonides Sleep Arts & Sciences, where the study was conducted in part; Barry and Jessica Krakow are married. Author BK reports: 6 main activities related to his work in sleep medicine. For websites, Dr. Krakow owns and operates 6 sites that provide education and offer products and services for sleep disorders patients: www.nightmaretreatment.com, www.ptsdsleepclinic.com, www.sleeptreatment.com, www.sleepdynamictherapy.com, www.soundsleepsoundmind.com, and www.nocturiacures.com. For other professional services, he is the medical director of a national DME company Classic SleepCare for which his sole functions are consultation and QA; he has neither patient encounters nor does he benefit from the sale of any DME equipment. For intellectual property, Dr. Krakow markets and sells 3 books for sleep disorders patients: Insomnia Cures, Turning Nightmares into Dreams, and Sound Sleep, Sound Mind. For clinical services, he owns and operates one commercial sleep center: Maimonides Sleep Arts & Sciences, Ltd. For educational and consulting services: Dr. Krakow conducts CME/CEU educational programs for medical and mental health providers to learn about sleep disorders. Sometimes these programs involve the attendee paying a fee directly to Maimonides Sleep Arts & Sciences. Other times, he conducts the workshops at other locations, which may be paid for by vendors such as Respironics and ResMed or other institutions such as the AMEDDC&S, VAMC, and regional sleep center conferences. He is also president and principal investigator of a non-profit sleep research center, the Sleep & Human Health Institute (www.sleepingresearch.org, www.shhi.org) that occasionally provides consultation services or receives grants for pilot studies, the most recent: ResMed ~$400,000 January 2015 (funding for this randomized control trial of treatment in insomnia patients). Recently, and after this research had been conducted, he provided a brief consultation to ASOCorp, a medical supply company that manufactures nasal strips.
Figures


References
-
- Morin C.M., Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. March 24. - PubMed
-
- Riemann D., Nissen C., Palagini L., Otte A., Perlis M.L., Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–558. May. - PubMed
-
- Morgenthaler T., Kramer M., Alessi C., Friedman L., Boehlecke B., Brown T. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. November. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

