Use of antenatal fluorinated corticosteroids in management of congenital heart block: Systematic review and meta-analysis
- PMID: 31517303
- PMCID: PMC6728741
- DOI: 10.1016/j.eurox.2019.100072
Use of antenatal fluorinated corticosteroids in management of congenital heart block: Systematic review and meta-analysis
Abstract
Objective: To evaluate outcomes of fluorinated corticosteroids, with or without other medications, for treatment of congenital heart block in-utero.
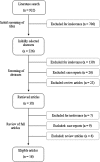
Study design: A search was conducted through MEDLINE, EMBASE, WEB OF SCIENCE and SCOPUS from inception to October 2017. Only comparative studies are considered eligible. Outcomes include fetal death, downgrade of heart block, neonatal death, need for neonatal pacing, fetal and maternal complications. Random effects model was used.
Results: Out of 923 articles, 12 studies were eligible. Compared to no treatment, there was no significant difference in incidence of fetal death (OR 1.10, 95%CI 0.65-1.84), neonatal death (OR 0.98, 95%CI 0.41-2.33), or need for pacing (OR 1.46, 95%CI 0.78-2.74). Heart block downgrade was significantly higher in treatment group (9.48%vs.1.76%, OR 3.27, 95%CI 1.23-8.71).
Conclusion: antenatal fluorinated corticosteroids do not improve fetal/neonatal morbidity or mortality of congenital heart block and are associated with higher incidence of fetal and maternal complications.
Keywords: Congenital heart disease; Fetal diagnosis; Fetal therapy; Prenatal treatment.
Figures



Similar articles
-
Maternal steroid therapy for fetuses with immune-mediated complete atrioventricular block: a systematic review and meta-analysis.J Matern Fetal Neonatal Med. 2019 Jun;32(11):1884-1892. doi: 10.1080/14767058.2017.1419182. Epub 2018 Oct 11. J Matern Fetal Neonatal Med. 2019. PMID: 29251180
-
No. 367-2019 Canadian Guideline for Physical Activity throughout Pregnancy.J Obstet Gynaecol Can. 2018 Nov;40(11):1528-1537. doi: 10.1016/j.jogc.2018.07.001. Epub 2018 Oct 5. J Obstet Gynaecol Can. 2018. PMID: 30297272
-
Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis.JAMA Pediatr. 2018 Jul 1;172(7):635-645. doi: 10.1001/jamapediatrics.2018.0302. JAMA Pediatr. 2018. PMID: 29813153 Free PMC article.
-
Antenatal Corticosteroids and Preterm Neonatal Morbidity and Mortality among Women with and without Diabetes in Pregnancy.Am J Perinatol. 2022 Jan;39(1):67-74. doi: 10.1055/s-0040-1714391. Epub 2020 Jul 27. Am J Perinatol. 2022. PMID: 32717749 Free PMC article.
-
Outcome of isolated congenital complete heart block diagnosed in utero.Heart. 1996 Feb;75(2):190-4. doi: 10.1136/hrt.75.2.190. Heart. 1996. PMID: 8673760 Free PMC article.
Cited by
-
Short and long-term outcomes of children with autoimmune congenital heart block treated with a combined maternal-neonatal therapy. A comparison study.J Perinatol. 2022 Sep;42(9):1161-1168. doi: 10.1038/s41372-022-01431-4. Epub 2022 Jun 18. J Perinatol. 2022. PMID: 35717457
-
British Society for Rheumatology guideline on management of adult and juvenile onset Sjögren disease.Rheumatology (Oxford). 2025 Feb 1;64(2):409-439. doi: 10.1093/rheumatology/keae152. Rheumatology (Oxford). 2025. PMID: 38621708 Free PMC article.
-
Obstetric management of the most common autoimmune diseases: A narrative review.Front Glob Womens Health. 2022 Nov 23;3:1031190. doi: 10.3389/fgwh.2022.1031190. eCollection 2022. Front Glob Womens Health. 2022. PMID: 36505012 Free PMC article. Review.
-
[Research progress on the manifestations and prognosis of neonatal lupus erythematosus in various systems].Zhongguo Dang Dai Er Ke Za Zhi. 2024 Jan 15;26(1):81-85. doi: 10.7499/j.issn.1008-8830.2306125. Zhongguo Dang Dai Er Ke Za Zhi. 2024. PMID: 38269464 Free PMC article. Review. Chinese.
-
Diagnosis and Management of Fetal Autoimmune Atrioventricular Block.Int J Womens Health. 2020 Aug 12;12:633-639. doi: 10.2147/IJWH.S257407. eCollection 2020. Int J Womens Health. 2020. PMID: 32884363 Free PMC article. Review.
References
-
- Yan J., Varma S.K., Malhotra A., Menahem S. Congenital complete heart block: single tertiary centre experience. Heart Lung Circ. 2012;21(11):666–670. - PubMed
-
- Brucato A., Frassi M., Franceschini F., Cimaz R., Faden D., Pisoni M.P., Muscarà M., Vignati G., Stramba‐Badiale M., Catelli L. Risk of congenital complete heart block in newborns of mothers with anti‐Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheumatol. 2001;44(8):1832–1835. - PubMed
-
- Grava C., Ruffatti A., Milanesi O., Favaro M., Tonello M., Calligaro A., Del Ross T., Todesco S. Isolated congenital heart block in undifferentiated connective tissue disease and in primary Sjögren’s syndrome: a clinical study of 81 pregnancies in 41 patients. Reumatismo. 2005;57(3):180–186. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

