Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration
- PMID: 31517483
- PMCID: PMC7220812
- DOI: 10.1021/acsami.9b07053
Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration
Abstract
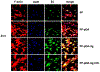
Biodegradable synthetic scaffolds hold great promise for oral and craniofacial guided tissue regeneration and bone regeneration. To overcome the limitations of current scaffold materials in terms of osteogenic and antimicrobial properties, we have developed a novel silver-modified/collagen-coated electrospun poly-lactic-co-glycolic acid/polycaprolactone (PLGA/PCL) scaffold (PP-pDA-Ag-COL) with improved antimicrobial and osteogenic properties. Our novel scaffold was generated by electrospinning a basic PLGA/PCL matrix, followed by silver nanoparticles (AgNPs) impregnation via in situ reduction, polydopamine coating, and then coating by collagen I. The three intermediate materials involved in the fabrication of our scaffolds, namely, PLGA/PCL (PP), PLGA/PCL-polydopamine (PP-pDA), and PLGA/PCL-polydopamine-Ag (PP-pDA-Ag), were used as control scaffolds. Scanning electron micrographs and mechanical testing indicated that the unique three-dimensional structures with randomly oriented nanofibrous electrospun scaffold architectures, the elasticity modulus, and the tensile strength were maintained after modifications. CCK-8 cell proliferation analysis demonstrated that the PP-pDA-Ag-COL scaffold was associated with higher MC3T3 proliferation rates than the three control scaffolds employed. Scanning electron and fluorescence light microscopy illustrated that PP-pDA-Ag-COL scaffolds significantly enhanced MC3T3 cell adhesion compared to the control scaffolds after 12 and 24 h culture, in tandem with the highest β1 integrin expression levels, both at the mRNA level and the protein level. Alkaline phosphatase activity, BMP2, and RUNX2 expression levels of MC3T3 cells cultured on PP-pDA-Ag-COL scaffolds for 7 and 14 days were also significantly higher when compared to controls (P < 0.001). There was a wider antibacterial zone associated in PP-pDA-Ag-COL and PP-pDA-Ag scaffolds versus control scaffolds (P < 0.05), and bacterial fluorescence was reduced on the Ag-modified scaffolds after 24 h inoculation against Staphylococcus aureus and Streptococcus mutans. In a mouse periodontal disease model, the PP-pDA-Ag-COL scaffold enhanced alveolar bone regeneration (31.8%) and was effective for periodontitis treatment. These results demonstrate that our novel PP-pDA-Ag-COL scaffold enhanced biocompatibility and osteogenic and antibacterial properties and has therapeutic potential for alveolar/craniofacial bone regeneration.
Keywords: antimicrobial; biomimetic scaffolds; electrospinning; local delivery; osteogenic; silver nanoparticle.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



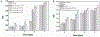







References
-
- Soldatos NK; Stylianou P; Koidou VP; Angelov N; Yukna R; Romanos GE Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48 (2), 131–147. - PubMed
-
- Qian YZ; Zhou XF; Sun H; Yang JX; Chen Y; Li C; Wang HJ; Xing T; Zhang FM; Gu N Biomimetic Domain-Active Electrospun Scaffolds Facilitating Bone Regeneration Synergistically with Antibacterial Efficacy for Bone Defects. ACS Appl. Mater. Interfaces 2018, 10 (4), 3248–3259, DOI: 10.1021/acsami.7b14524. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

