De novo phosphatidylcholine synthesis is required for autophagosome membrane formation and maintenance during autophagy
- PMID: 31517566
- PMCID: PMC7469489
- DOI: 10.1080/15548627.2019.1659608
De novo phosphatidylcholine synthesis is required for autophagosome membrane formation and maintenance during autophagy
Erratum in
-
Correction.Autophagy. 2025 Mar;21(3):iii. doi: 10.1080/15548627.2024.2431342. Epub 2025 Jan 15. Autophagy. 2025. PMID: 39814343 Free PMC article. No abstract available.
Abstract
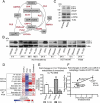
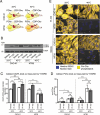
Macroautophagy/autophagy can enable cancer cells to withstand cellular stress and maintain bioenergetic homeostasis by sequestering cellular components into newly formed double-membrane vesicles destined for lysosomal degradation, potentially affecting the efficacy of anti-cancer treatments. Using 13C-labeled choline and 13C-magnetic resonance spectroscopy and western blotting, we show increased de novo choline phospholipid (ChoPL) production and activation of PCYT1A (phosphate cytidylyltransferase 1, choline, alpha), the rate-limiting enzyme of phosphatidylcholine (PtdCho) synthesis, during autophagy. We also discovered that the loss of PCYT1A activity results in compromised autophagosome formation and maintenance in autophagic cells. Direct tracing of ChoPLs with fluorescence and immunogold labeling imaging revealed the incorporation of newly synthesized ChoPLs into autophagosomal membranes, endoplasmic reticulum (ER) and mitochondria during anticancer drug-induced autophagy. Significant increase in the colocalization of fluorescence signals from the newly synthesized ChoPLs and mCherry-MAP1LC3/LC3 (microtubule-associated protein 1 light chain 3) was also found on autophagosomes accumulating in cells treated with autophagy-modulating compounds. Interestingly, cells undergoing active autophagy had an altered ChoPL profile, with longer and more unsaturated fatty acid/alcohol chains detected. Our data suggest that de novo synthesis may be required to increase autophagosomal ChoPL content and alter its composition, together with replacing phospholipids consumed from other organelles during autophagosome formation and turnover. This addiction to de novo ChoPL synthesis and the critical role of PCYT1A may lead to development of agents targeting autophagy-induced drug resistance. In addition, fluorescence imaging of choline phospholipids could provide a useful way to visualize autophagosomes in cells and tissues.
Abbreviations: AKT: AKT serine/threonine kinase; BAX: BCL2 associated X, apoptosis regulator; BECN1: beclin 1; ChoPL: choline phospholipid; CHKA: choline kinase alpha; CHPT1: choline phosphotransferase 1; CTCF: corrected total cell fluorescence; CTP: cytidine-5'-triphosphate; DCA: dichloroacetate; DMEM: dulbeccos modified Eagles medium; DMSO: dimethyl sulfoxide; EDTA: ethylenediaminetetraacetic acid; ER: endoplasmic reticulum; GDPD5: glycerophosphodiester phosphodiesterase domain containing 5; GFP: green fluorescent protein; GPC: glycerophosphorylcholine; HBSS: hanks balances salt solution; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; LPCAT1: lysophosphatidylcholine acyltransferase 1; LysoPtdCho: lysophosphatidylcholine; MRS: magnetic resonance spectroscopy; MTORC1: mechanistic target of rapamycin kinase complex 1; PCho: phosphocholine; PCYT: choline phosphate cytidylyltransferase; PLA2: phospholipase A2; PLB: phospholipase B; PLC: phospholipase C; PLD: phospholipase D; PCYT1A: phosphate cytidylyltransferase 1, choline, alpha; PI3K: phosphoinositide-3-kinase; pMAFs: pancreatic mouse adult fibroblasts; PNPLA6: patatin like phospholipase domain containing 6; Pro-Cho: propargylcholine; Pro-ChoPLs: propargylcholine phospholipids; PtdCho: phosphatidylcholine; PtdEth: phosphatidylethanolamine; PtdIns3P: phosphatidylinositol-3-phosphate; RPS6: ribosomal protein S6; SCD: stearoyl-CoA desaturase; SEM: standard error of the mean; SM: sphingomyelin; SMPD1/SMase: sphingomyelin phosphodiesterase 1, acid lysosomal; SGMS: sphingomyelin synthase; WT: wild-type.
Keywords: Autophagosome; CTP:phosphocholine cytidylyltransferase; autophagy; choline phospholipids; phosphatidylcholine; propargylcholine.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures






References
-
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. PMID:21801009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
