Research Lifecycle to Increase the Substantial Real-world Impact of Research: Accelerating Innovations to Application
- PMID: 31517789
- PMCID: PMC6750195
- DOI: 10.1097/MLR.0000000000001146
Research Lifecycle to Increase the Substantial Real-world Impact of Research: Accelerating Innovations to Application
Abstract
Background: US health care systems face a growing demand to incorporate innovations that improve patient outcomes at a lower cost. Funding agencies increasingly must demonstrate the impact of research investments on public health. The Learning Health System promotes continuous institutional innovation, yet specific processes to develop innovations for further research and implementation into real-world health care settings to maximize health impacts have not been specified.
Objective: We describe the Research Lifecycle and how it leverages institutional priorities to support the translation of research discoveries to clinical application, serving as a broader operational approach to enhance the Learning Health System.
Methods: Developed by the US Department of Veterans Affairs Office of Research and Development Research-to-Real-World Workgroup, the Research Lifecycle incorporates frameworks from product development, translational science, and implementation science methods. The Lifecycle is based on Workgroup recommendations to overcome barriers to more direct translation of innovations to clinical application and support practice implementation and sustainability.
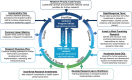
Results: The Research Lifecycle posits 5 phases which support a seamless pathway from discovery to implementation: prioritization (leadership priority alignment), discovery (innovation development), validation (clinical, operational feasibility), scale-up and spread (implementation strategies, performance monitoring), and sustainability (business case, workforce training). An example of how the Research Lifecycle has been applied within a health system is provided.
Conclusions: The Research Lifecycle aligns research and health system investments to maximize real-world practice impact via a feasible pathway, where priority-driven innovations are adapted for effective clinical use and supported through implementation strategies, leading to continuous improvement in real-world health care.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Balas EA, Boren SA. Managing clinical knowledge for health care improvement. Yearb Med Inform. 2000;1:65–70. - PubMed
-
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374:86–89. - PubMed
-
- Ferguson TB., Jr The Institute of Medicine committee report “best care at lower cost: the path to continuously learning health care”. Circ Cardiovasc Qual Outcomes. 2012;5:e93–e94. - PubMed
-
- Bindman AB, Pronovost PJ, Asch DA. Funding innovation in a learning health care system. JAMA. 2018;319:119–120. - PubMed
-
- Whicher D, Rosengren K, Siddiqi S, Simpson L, The Future of Health Services Research: Advancing Health Systems Research and Practice in the United States. Washington, DC: National Academy of Medicine; 2018. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical