Effects of dangguijakyaksan on lower-extremity blood circulation disturbances in climacteric and postmenopausal women: Study protocol for a randomized, double-blind, placebo-controlled pilot trial
- PMID: 31517823
- PMCID: PMC6750319
- DOI: 10.1097/MD.0000000000017039
Effects of dangguijakyaksan on lower-extremity blood circulation disturbances in climacteric and postmenopausal women: Study protocol for a randomized, double-blind, placebo-controlled pilot trial
Abstract
Background: Climacteric women experience various disorders, including hot flush, depression, insomnia, arthralgia, and hand and foot numbness. Dangguijakyaksan is among the most common treatments for climacteric syndrome, and its effect on depression, insomnia, hot flush and quality of life (QOL) in climacteric women has been reported multiple times. A recent animal study found dangguijakyaksan decreased serum lipid factors and improved blood circulation in a menopausal rat model; however, these effects have not been assessed in clinical trials. This study aims to assess the clinical effects and safety of dangguijakyaksan for lower-extremity blood circulation disturbances in climacteric women.
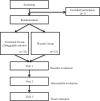
Methods: This is a single-center, randomized, double-blinded, placebo-controlled pilot study that will be conducted at Dunsan Korean Medicine Hospital at Daejeon University. Forty-six climacteric women with lower-extremity blood circulation disturbances will be recruited and randomized (1:1) into either the dangguijakyaksan or placebo group. After 8 weeks of administration, the effects and safety of dangguijakyaksan will be assessed.The primary outcome is the visual analogue scale for lower-extremity blood circulation disturbances, and it will be assessed on visits 1, 2, and 3. The secondary outcomes, Kupperman's index and blood deficiency scoring system, will be assessed on visits 1, 2, and 3, and accelerated photoplethysmography and digital infrared thermal imaging will be performed on visits 1 and 3. Moreover, blood lipid profile, follicle-stimulating hormone, and estradiol levels will be measured at the screening visit and visit 3. Blood tests will be performed at the screening visit and visit 3 to assess the safety of dangguijakyaksan. Statistical analysis will be performed using R-3.3.3 (Another Canoe), and within-group study variable differences after drug administration will be analyzed using paired t-test or Wilcoxon signed-rank test.
Discussion: We expect to confirm the effects and safety of dangguijakyaksan on lower-extremity blood circulation disturbances in menopause, which would provide foundational data for planning subsequent studies.
Conflict of interest statement
The authors have no conflicts of interests to disclose.
Figures
References
-
- Obstetrics and Gynecology Society Textbook Compilation Committee. Gynecology. Seoul: Korea Medical Book Publishing Company; 2015.
-
- NICE, NICE Qulitiy standard, Menopause. 9 February 2017. Available at: https://www.nice.org.uk/guidance/qs143 Accessed May 20, 2019.
-
- Huang K-E, Xu L, Nasri N, et al. The Asian Menopause Survey: knowledge, perceptions, hormone treatment and sexual function. Maturitas 2010;65:276–83. - PubMed
-
- Pinkerton JAV, Aguirre FS, Blake J, et al. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause 2017;24:728–53. - PubMed
-
- Sun SK, Song HY, Kim JS, et al. Decisional conflict about hormone replacement therapy in postmenopausal women and its related factors. J Korean Acad Fam Med 2006;27:629–36.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources