Topical cyclosporine A therapy for dry eye syndrome
- PMID: 31517988
- PMCID: PMC6743670
- DOI: 10.1002/14651858.CD010051.pub2
Topical cyclosporine A therapy for dry eye syndrome
Abstract
Background: Topical cyclosporine A (also known as ciclosporin A) (CsA) is an anti-inflammatory that has been widely used to treat inflammatory ocular surface diseases. Two CsA eyedrops have been approved by US Food and Drug Administration for managing dry eye: Restasis (CsA 0.05%, Allergan Inc, Irvine, CA, USA), approved in 2002, and Cequa (CsA 0.09%, Sun Pharma, Cranbury, NJ, USA), approved in 2018. Numerous clinical trials have been performed to assess the effectiveness and safety of CsA for dry eye; however, there is no universal consensus with regard to its effect.
Objectives: To assess the effectiveness and safety of topical CsA in the treatment of dry eye.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2018, Issue 2); Ovid MEDLINE; Embase.com; PubMed; Latin American and Caribbean Health Sciences Literature Database (LILACS); ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 16 February 2018.
Selection criteria: We included randomized controlled trials (RCTs) of people with dry eye regardless of age, sex, severity, etiology, or classification of dry eye. We included RCTs in which different concentrations of topical CsA were compared with one another or with artificial tears, placebo, or vehicle. We also included RCTs in which CsA in combination with artificial tears was compared to artificial tears alone.
Data collection and analysis: We followed the standard Cochrane methodology and assessed the certainty of the evidence using GRADE.
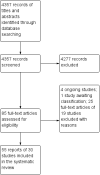
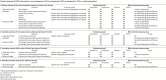
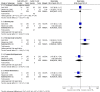
Main results: We included 30 RCTs (4009 participants) with follow-up periods ranging from 6 weeks to 12 months. We studied dry eye of various severity and underlying causes. The interventions investigated also varied across RCTs: CsA versus artificial tears; CsA with artificial tears versus artificial tears alone; and in some studies, more than one concentration of CsA. Artificial tears were used as adjunctive to study medication in all but five trials. Almost all trials had deficiencies in the reporting of results (e.g. reporting P values or direction only), precluding the calculation of between-group estimates of effect or meta-analysis.Eighteen trials compared topical CsA 0.05% plus artificial tears versus vehicle plus artificial tears or artificial tears alone. One trial reported subjective symptoms of dry eye at 6 months and the results were in favor of CsA (mean difference (MD) -4.80, 95% confidence interval (CI) -6.41 to -3.19; low-certainty evidence). Two trials reported MD in ocular surface dye staining at 6 months, but the results were inconsistent in these two trials (MD -0.35, 95% CI -0.69 to -0.01 in one and MD 0.58, 95% CI 0.06 to 1.10 in the other; low-certainty evidence). Four trials reported MD in Schirmer test scores at 6 months and the estimates ranged from -4.05 (95% CI -6.67 to -1.73) to 3.26 (95% CI -1.52 to 5.00) (low-certainty evidence). Three trials reported risk ratio (RR) of improved Schirmer test scores at 6 months; estimates ranged from 0.98 (95% CI 0.83 to 1.17) to 3.50 (95% CI 2.09 to 5.85) (low-certainty evidence). Four trials reported MD in tear film stability measured by tear break-up time at 6 months and the estimates ranged from -1.98 (95% CI -3.59 to -0.37) to 1.90 (95% CI 1.44 to 2.36) (low-certainty evidence). Three trials reported RR of improved tear break-up time at 6 months and the estimates ranged from 0.90 (95% CI 0.77 to 1.04) to 4.00 (95% CI 2.25 to 7.12) (low-certainty evidence). Three trials reported frequency of artificial tear usage at 6 months without providing any estimates of effect; the direction of effect seem to be in favor of CsA (low-certainty evidence). Because of incomplete reporting of the results data or considerable statistical heterogeneity, we were only able to perform a meta-analysis on mean conjunctival goblet cell density. Mean conjunctival goblet cell density in the CsA treated group may be greater than that in the control group at the end of follow-up at four and 12 months (MD 22.5 cells per unit, 95% CI 16.3 to 28.8; low-certainty evidence). All but two trials reported adverse events that included burning and stinging. Participants treated with CsA may be more likely to have treatment-related adverse events than those who treated with vehicle (RR 1.33, 95% CI 1.00 to 1.78; low-certainty evidence).Other comparisons evaluated were CsA 0.05% plus artificial tears versus higher concentrations of CsA plus artificial tears (4 trials); CsA 0.05% versus placebo or vehicle (4 trials); CsA 0.1% plus artificial tears versus placebo or vehicle plus artificial tears (2 trials);CsA 0.1% cationic emulsion plus artificial tears versus vehicle plus artificial tears (2 trials); CsA 1% plus artificial tears versus placebo plus artificial tears (3 trials); and CsA 2% plus artificial tears versus placebo plus artificial tears (3 trials). Almost all of these trials reported P value or direction of effect only (mostly in favor of CsA), precluding calculation of between-group effect estimates or meta-analyses.
Authors' conclusions: Despite the widespread use of topical CsA to treat dry eye, we found that evidence on the effect of CsA on ocular discomfort and ocular surface and tear film parameters such as corneal fluorescein staining, Schirmer's test, and TBUT is inconsistent and sometimes may not be different from vehicle or artificial tears for the time periods reported in the trials. There may be an increase in non-serious, treatment-related adverse effects (particularly burning) in the CsA group. Topical CsA may increase the number of conjunctival goblet cells. However, current evidence does not support that improvements in conjunctival mucus production (through increased conjunctival goblet cells) translate to improved symptoms or ocular surface and tear film parameters. All published trials were short term and did not assess whether CsA has longer-term disease-modifying effects. Well-planned, long-term, large clinical trials are needed to better assess CsA on long-term dry eye-modifying effects. A core outcome set, which ideally includes both biomarkers and patient-reported outcomes in the field of dry eye, is needed.
Conflict of interest statement
CSDP: none. SCP: Allergan (Consultant, research); Senju (Consultant); Shire (Consultant, research). SN: none. EKA: KeraLink (National Medical Director); Novaliq, Novartis Pharma AG, Shire, Regenero, Sun Ophthalmics (Consultant); unpaid member of Board of Directors Sjogren's Foundation, Cornea Society; Allergan (grant support); W. L. Gore & Associates (royalties).
Figures














Update of
References
References to studies included in this review
Altiparmak 2010 {published data only}
-
- Altiparmak UE, Acar DE, Ozer PA, Emec SD, Kasim R, Ustun H, et al. Topical cyclosporine A for the dry eye findings of thyroid orbitopathy patients. Eye (London, England) 2010;24(6):1044‐50. - PubMed
Baiza‐Durán 2010 {published data only}
-
- Baiza‐Durán L, Medrano‐Palafox J, Hernández‐Quintela E, Lozano‐Alcazar J, Alaníz‐de la OJF. A comparative clinical trial of the efficacy of two different aqueous solutions of cyclosporine for the treatment of moderate‐to‐severe dry eye syndrome. British Journal of Ophthalmology 2010;94(10):1312‐5. - PubMed
Barreto 2009 {published data only}
-
- Barreto RPP, Biancardi AL, Nascimento EM, Pereira BB, Moraes JHV. Topical cyclosporine 0.05% for the treatment of dry eye disease in patients infected with the human immunodeficiency virus [Uso de ciclosporina 0.05% tópica no tratamento do olho seco de pacientes portadores do vírus HIV]. Revista Brasileira de Oftalmologia 2009;68(2):83‐9. [NCT00797030]
Baudouin 2017 {published data only}
-
- Baudouin C, Figueiredo FC, Messmer EM, Ismail D, Amrane M, Garrigue J, et al. A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in treatment of moderate to severe dry eye. European Journal of Ophthalmology 2017;27(5):520‐30. [EudraCT database under number 2007‐000029‐23 with the protocol code NVG06C103] - PMC - PubMed
Brignole 2001 {published data only}
-
- Baudouin AC, Brignole F, Pisella PJ, Saint Jean M, Goguel A. Expression of inflammatory markers in dry eye syndrome following 12 months' treatment with topical cyclosporin. Investigative Ophthalmology & Visual Science 2001;42:ARVO abstract 4942. - PubMed
-
- Baudouin AC, Brignole F, Pisella PJ, Goldschild M, Saint Jean M, Goguel A. Flow cytometric analysis of inflammatory markers in dry eyes: results of a six‐month treatment with topical ciclosporin. Investigative Ophthalmology & Visual Science 2000;41:ARVO abstract 3567.
-
- Baudouin C, Brignole F, Pisella PJ, Jean MS, Goguel A. Flow cytometric analysis of the inflammatory marker HLA DR in dry eye syndrome: results from 12 months of randomized treatment with topical cyclosporin A. Advances in Experimental Medicine and Biology 2002;506(Pt B):761‐9. - PubMed
-
- Brignole F, Pisella PJ, Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6‐month treatment with topical cyclosporin A. Investigative Ophthalmology & Visual Science 2001;42(1):90‐5. [Protocol 192371‐501‐03 designed by Allergan Inc.] - PubMed
-
- Galatoire O, Baudouin C, Pisella PJ, Brignole F. Flow cytometry in impression cytology during keratoconjunctivitis sicca: effects of topical cyclosporin A on HLA DR expression. Journal Francais d'Ophtalmologie 2003;26(4):337‐43. - PubMed
Chen 2010 {published data only}
-
- Chen M, Gong L, Sun X, Xie H, Zhang Y, Zou L, et al. A comparison of cyclosporine 0.05% ophthalmic emulsion versus vehicle in Chinese patients with moderate to severe dry eye disease: an eight‐week, multicenter, randomized, double‐blind, parallel‐group trial. Journal of Ocular Pharmacology and Therapeutics 2010;26(4):361‐6. - PubMed
Chung 2013 {published data only}
Demiryay 2011 {published data only}
-
- Demiryay E, Yaylali V, Cetin EN, Yildirim C. Effects of topical cyclosporine A plus artificial tears versus artificial tears treatment on conjunctival goblet cell density in dysfunctional tear syndrome. Eye & Contact Lens 2011;37(5):312‐5. - PubMed
Fan 2003 {published data only}
-
- Fan WS, Hung HL, Liao HP, Lai NS. Topical cyclosporine therapy for keratoconjunctivitis sicca in Sjogrens' syndrome. Tzu Chi Medical Journal 2003;15(2):85‐9.
Foulks 1996 {published data only}
-
- Foulks G, Pflugfelder S, Lemp M, Stern K, Burk C, Reis B. A randomized double‐masked clinical trial to assess efficacy and safety of a 1% CsA‐containing ophthalmic ointment vs. placebo in patients with keratoconjunctivitis sicca (KCS) associated with Sjögren's syndrome (SS). Investigative Ophthalmology & Visual Science 1996;37:ARVO abstract 2992.
Gündüz 1994 {published data only}
-
- Gündüz K, Ozdemir O. Topical cyclosporin treatment of keratoconjunctivitis sicca in secondary Sjögren's syndrome. Acta Ophthalmologica 1994;72(4):438‐42. - PubMed
Guzey 2009 {published data only}
-
- Guzey M, Karaman SK, Satici A, Ozardali I, Sezer S, Bozkurt O. Efficacy of topical cyclosporine A in the treatment of severe trachomatous dry eye. Clinical & Experimental Ophthalmology 2009;37(6):541‐9. - PubMed
-
- Guzey M, Satici A, Karaman SK, Ordulu F, Sezer S. The effect of topical cyclosporine A treatment on corneal thickness in patients with trachomatous dry eye. Clinical & Experimental Optometry 2009;92(4):349‐55. - PubMed
Helms 1996 {published data only}
-
- Helms H, Rapoza P, Stern K, Burk C, Rosenthal A, Reis B. A randomized double‐masked clinical trial to assess the safety and comparative efficacy of three concentrations of a cyclosporine‐containing ophthalmic ointment vs. placebo in patients with keratoconjunctivitis sicca (KCS). Investigative Ophthalmology & Visual Science 1996;37:ARVO abstract 2993.
Jain 2007 {published data only}
-
- Jain AK, Sukhija J, Dwedi S, Sood A. Effect of topical cyclosporine on tear functions in tear‐deficient dry eyes. Annals of Ophthalmology (Skokie, Ill.) 2007;39(1):19‐25. - PubMed
Kim 2009 {published data only}
-
- Kim EC, Choi JS, Joo CK. A comparison of vitamin A and cyclosporine A 0.05% eye drops for treatment of dry eye syndrome. American Journal of Ophthalmology 2009;147(2):206‐13. [ISRCTN 47762748] - PubMed
Laibovitz 1993 {published data only}
-
- Laibovitz RA, Solch S, Andriano K, O'Connell M, Silverman MH. Pilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca. Cornea 1993;12(4):315‐23. - PubMed
Lankaranian 2006 {published data only}
-
- Lankaranian D, Patel R, Moster M, Wizov S, Alvim HS, Lopes JF, et al. A randomized prospective clinical trial of the efficacy of cyclosporine ophthalmic emulsion 0.05% following trabeculectomy with antimetabolite. Investigative Ophthalmology & Visual Science 2006;47:ARVO abstract 5486.
Leonardi 2016 {published data only}
-
- Leonardi A, Setten G, Amrane M, Ismail D, Garrigue J, Figueiredo FC, et al. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. European Journal of Ophthalmology 2016;26(4):287‐96. [EudraCT database: 2011‐000160‐97 with the protocol code number NVG10E117 ] - PubMed
Liew 2012 {published data only}
-
- Huang JF, Yafawi R, Zhang M, McDowell M, Rittenhouse KD, Sace F, et al. Immunomodulatory effect of the topical ophthalmic Janus kinase inhibitor tofacitinib (CP‐690,550) in patients with dry eye disease. Ophthalmology 2012;119(7):e43‐50. - PubMed
-
- Liew SH, Nichols KK, Klamerus KJ, Li JZ, Zhang M, Foulks GN. Tofacitinib (CP‐690,550), a Janus kinase inhibitor for dry eye disease: results from a phase 1/2 trial. Ophthalmology 2012;119(7):1328‐35. [NCT00784719] - PubMed
Lopez 2006 {published data only}
-
- Lopez R. Topical 0.05% cyclosporine: effect on the quality of life of dry eye patients. Optometry 2006;77:296.
Ma 2015 {published data only}
-
- Ma K, Lyu Z, Liao J, Wang S, Deng Y. Efficacy and safety of 0.05% cyclosporine A ophthalmic emulsion in treatment of dry eye. Zhonghua Shiyan Yanke Zazhi [Chinese Journal of Experimental Ophthalmology] 2015;33(7):655‐9.
Prabhasawat 2012 {published data only}
-
- Prabhasawat P, Tesavibul N, Mahawong W. A randomized double‐masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea 2012;31:1386‐93. [NCT00705510] - PubMed
Rao 2010 {published data only}
-
- Asano K, Rao S. Topical cyclosporine for the prevention of dry eye disease: second year of two‐year study. American Academy of Optometry. Orlando (FL), 2009. [95707]
-
- Rao S. Prevention of dry eye disease progression by topical cyclosporine 0.05%. American Academy of Ophthalmology. San Francisco (CA), 2008:202. [PO078]
-
- Rao SN. Reversibility of dry eye deceleration after topical cyclosporine 0.05% withdrawal. Journal of Ocular Pharmacology & Therapeutics 2011;27(6):603‐9. - PubMed
-
- Rao SN. The impact of topical cyclosporine 0.05% on dry eye and daily activity. Investigative Ophthalmology & Visual Science 2006;47:ARVO abstract 263.
-
- Rao SN. Topical cyclosporine 0.05% for the prevention of dry eye disease progression. Investigative Ophthalmology & Visual Science 2008;49:ARVO abstract 100.
Salib 2006 {published data only}
-
- Salib GM, McDonald MB, Smolek M. Safety and efficacy of cyclosporine 0.05% drops versus unpreserved artificial tears in dry‐eye patients having laser in situ keratomileusis. Journal of Cataract and Refractive Surgery 2006;32(5):772‐8. - PubMed
Sall 2000 {published data only}
-
- Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology 2005;112(10):1790‐4. - PubMed
-
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Archives of Ophthalmology 2002;120(3):330‐7. - PubMed
-
- Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Archives of Ophthalmology 2000;118(11):1489‐96. - PubMed
-
- Sall K. Erratum: Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye diseases (Ophthalmology (2000) 107 (631‐9)). Ophthalmology 2000; Vol. 107, issue 7:1220. - PubMed
-
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology 2000;107(4):631‐9. - PubMed
Sall 2006 {published data only}
-
- Christensen M, Hearn C, Tudor M, Stein J, Meadows D, Stone R, et al. Efficacy of a cyclosporine‐based dry eye therapy with two marketed artificial tears as supportive therapy. American Academy of Optometry. Orlando (FL), 2004. [Poster 7]
-
- Sall KN, Cohen SM, Christensen MT. Efficacy and compatibility of an HP‐guar based lubricant eye drop when used as supportive therapy with a cyclosporine‐based ophthalmic emulsion. Investigative Ophthalmology & Visual Science 2005;46:ARVO abstract 2020.
-
- Sall KN, Cohen SM, Christensen MT, Stein JM. An evaluation of the efficacy of a cyclosporine‐based dry eye therapy when used with marketed artificial tears as supportive therapy in dry eye. Eye & Contact Lens 2006;32(1):21‐6. - PubMed
Schrell 2012 {published data only}
-
- Schrell C, Cursiefen C, Kruse F, Jacobi C. Topical cyclosporine A 0.05% in the treatment of keratoconjunctivitis sicca. Klinische Monatsblatter fur Augenheilkunde 2012;229(5):548‐53. - PubMed
Stevenson 2000 {published data only}
-
- Reis BL, Burk CT, Stern KL. The safety and efficacy of cyclosporine (0.05, 0.1, 0.2 and 0.4%) and vehicle ophthalmic emulsions in the treatment of moderate to severe keratoconjunctivitis sicca (KCS). Investigative Ophthalmology & Visual Science 1997;38:ARVO abstract 1024.
-
- Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate‐to‐severe dry eye disease: a dose‐ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology 2000;107(5):967‐74. - PubMed
-
- Tauber J. A dose‐ranging clinical trial to assess the safety and efficacy of cyclosporine ophthalmic emulsion in patients with keratoconjunctivitis sicca. The Cyclosporine Study Group. Advances in Experimental Medicine and Biology 1998;438:969‐72. - PubMed
Willen 2008 {published data only}
-
- Willen CM, McGwin G, Liu B, Owsley C, Rosenstiel C. Efficacy of cyclosporine 0.05% ophthalmic emulsion in contact lens wearers with dry eyes. Eye & Contact Lens 2008;34(1):43‐5. - PubMed
Wu 2009 {published data only}
-
- Wu AH, Wang BL, Pei S. Effect of cyclosporin A in the treatment of severe dry eye syndrome. International Journal of Ophthalmology 2009;9(2):389‐90.
References to studies excluded from this review
Arman 2015 {published data only}
Boynton 2015 {published data only}
-
- Boynton GE, Raoof D, Niziol LM, Hussain M, Mian SI. Prospective randomized trial comparing efficacy of topical loteprednol etabonate 0.5% versus cyclosporine‐A 0.05% for treatment of dry eye syndrome following hematopoietic stem cell transplantation. Cornea 2015;34(7):725‐32. - PubMed
Brown 2009 {published data only}
-
- Brown GC, Brown MM, Brown HC, Peet J, Roth Z. Topical cyclosporine (Restasis) cost‐utility analysis. Evidence‐Based Ophthalmology 2009;10(3):166‐71.
-
- Brown MM, Brown GC, Brown HC, Peet J, Roth Z. Value‐based medicine, comparative effectiveness, and cost‐effectiveness analysis of topical cyclosporine for the treatment of dry eye syndrome. Archives of Ophthalmology 2009;127(2):146‐52. - PubMed
Deveci 2014 {published data only}
-
- Deveci H, Kobak S. The efficacy of topical 0.05% cyclosporine A in patients with dry eye disease associated with Sjogren's syndrome. International Ophthalmology 2014;34(5):1043‐8. - PubMed
Donnenfeld 2007 {published data only}
-
- Donnenfeld E, Roberts C, Perry H, Solomon R, Wittpenn J, McDonald M. Efficacy of topical cyclosporine versus tears for improving visual outcomes following multifocal IOL implantation. Investigative Ophthalmology & Visual Science 2007;48:E‐Abstract 1066.
Donnenfeld 2010 {published data only}
-
- Donnenfeld ED, Solomon R, Roberts CW, Wittpenn JR, McDonald MB, Perry HD. Cyclosporine 0.05% to improve visual outcomes after multifocal intraocular lens implantation. Journal of Cataract and Refractive Surgery 2010;36(7):1095‐100. - PubMed
Hessert 2013 {published data only}
-
- Hessert D, Tanzer D, Brunstetter T, Kaupp S, Murdoch D, Mirzaoff M. Topical cyclosporine A for postoperative photorefractive keratectomy and laser in situ keratomileusis. Journal of Cataract and Refractive Surgery 2013;39(4):539‐47. - PubMed
Hom 2006 {published data only}
-
- Hom M. Comparison of cyclosporine A vs. rewetter for CL intolerance: a pilot study. American Academy of Optometry. Orlando (FL), 2004. [Poster 1]
-
- Hom MM. Use of cyclosporine 0.05% ophthalmic emulsion for contact lens‐intolerant patients. Eye & Contact Lens 2006;32(2):109‐11. - PubMed
Lee 2014 {published data only}
-
- Lee HS, Ji YS, Yoon KC. Efficacy of hypotonic 0.18% sodium hyaluronate eye drops in patients with dry eye disease. Cornea 2014;33(9):946‐51. - PubMed
Lee 2016 {published data only}
Lin 2015 {published data only}
Moon 2007 {published data only}
Roberts 2007a {published data only}
-
- Roberts C, Carniglia P, Brazzo B. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. American Academy of Optometry. Orlando (FL), 2005. [050070] - PubMed
-
- Roberts CW, Carniglia PE, Brazzo BG. Comparison of cyclosporine to punctal plugs in relieving the signs and symptoms of dry eyes. Investigative Ophthalmology & Visual Science 2005;46:ARVO abstract 2027.
-
- Roberts CW, Carniglia PE, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea 2007;26(7):805‐9. - PubMed
Roberts 2007b {published data only}
-
- Roberts CW. Dry eye symptoms following cataract surgery. Investigative Ophthalmology & Visual Science 2006;47:ARVO abstract 232.
-
- Roberts CW, Elie ER. Dry eye symptoms following cataract surgery. Insight (American Society of Ophthalmic Registered Nurses) 2007;32(1):14‐21. - PubMed
Schechter 2006 {published data only}
-
- Schechter B. Efficacy of topical cyclosporine 0.05% in the prevention of ocular surface inflammation secondary to pterygia. American Academy of Ophthalmology. San Francisco (CA), 2006:215. [PO081]
Su 2011 {published data only}
-
- Perry HD, Donnenfeld ED, Manris PJG. The effect of decreasing cyclosporine 0.05% on dry eye disease after one year of therapy. American Academy of Ophthalmology. San Francisco (CA), 2006:219. [PO435]
-
- Su MY, Perry HD, Barsam A, Perry AR, Donnenfeld ED, Wittpenn JR, et al. The effect of decreasing the dosage of cyclosporine A 0.05% on dry eye disease after 1 year of twice‐daily therapy. Cornea 2011;30(10):1098‐104. - PubMed
-
- Su MY, Perry HD, Donnenfeld ED, Wittpenn JR. The effect of decreasing cyclosporine 0.05% on dry eye disease after one year of therapy. Investigative Ophthalmology & Visual Science 2007;48:ARVO abstract 374.
Wan 2012 {published data only}
-
- Wan PX, Wang XR, Song YY, Li ZY, Duan HC, Zhang W, et al. Study on the treatment of dry eye with Loteprednol Etabonate. Chung‐Hua Yen Ko Tsa Chih [Chinese Journal of Ophthalmology] 2012;48(2):142‐7. - PubMed
Wang 2008 {published data only}
-
- Wang Y, Ogawa Y, Dogru M, Kawai M, Tatematsu Y, Uchino M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft‐versus‐host disease. Bone Marrow Transplantation 2008;41(3):293‐302. - PubMed
Wittpenn 2005 {published data only}
-
- Wittpenn JR, Schechter B. Efficacy of cyclosporine A for the treatment of ocular rosacea. Investigative Ophthalmology & Visual Science 2005;46:E‐abstract 2846.
References to studies awaiting assessment
Schultze 2004 {published data only}
-
- Schultze RL, Michra G. Comparison of topical cyclosporine (Restasis) and punctal occlusion in the treatment of dry eye syndrome. American Academy of Ophthalmology. San Francisco (CA), 2004:181. [PO229]
References to ongoing studies
KCT0002180 {unpublished data only}
-
- A multicenter, randomized, partial double blind, active control, parallel clinical trial to evaluate the efficacy, safety and usability of cyclosporin ophthalmic nanoemulsion 0.05% compared with cyclosporin ophthalmic emulsion 0.05% and diquafosol ophthalmic solution 3%. Ongoing study October 2016 (first enrollment).
NCT02688556 {unpublished data only}
-
- A randomized, multicenter, double‐masked, vehicle‐controlled study of the safety and efficacy of OTX‐101 in the treatment of keratoconjunctivitis sicca. Ongoing study February 2016.
NCT02917512 {unpublished data only}
-
- A multicenter, placebo controlled, Restasis referenced, randomized, double blind, Phase II study to evaluate the efficacy and safety of HU00701/HU007 eye drops in patients with dry eye syndrome. Ongoing study March 2016.
NCT03292809 {unpublished data only}
-
- CyclASol for the treatment of signs and symptoms of dry eye disease (DED). Ongoing study October 2017.
Additional references
Altinors 2006
-
- Altinors DD, Akça S, Akova YA, Bilezikçi B, Goto E, Dogru M, et al. Smoking associated with damage to the lipid layer of the ocular surface. American Journal of Ophthalmology 2006;141(6):1016‐21. - PubMed
De Paiva 2011
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Galatoire 2003
-
- Galatoire O, Baudouin C, Pisella PJ, Brignole F. Flow cytometry in impression cytology during keratoconjunctivitis sicca: effects of topical cyclosporin A on HLA DR expression [Cytofluorimetrie sur empreintes conjonctivales au cours de la keratoconjonctivite seche: effets de la ciclosporine topique sur l'expression d'antigene HLA DR]. Journal Francais d'Ophtalmologie 2003;26(4):337‐43. [PUBMED: 12843889] - PubMed
Glanville 2006
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Karakus 2018
-
- Karakus S, Agrawal D, Hindman HB, Henrich C, Ramulu PY, Akpek EK. Effects of Prolonged Reading on Dry Eye. Ophthalmology 2018;125(10):1500‐5. - PubMed
Kunert 2000
-
- Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Archives of Ophthalmology 2000;118(11):1489‐96. - PubMed
Kunert 2002
-
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Archives of Ophthalmology 2002;120(3):330‐7. - PubMed
Larson 2011
Li 2004
Mathews 2017
-
- Mathews PM, Ramulu PY, Swenor BS, Utine CA, Rubin GS, Akpek EK. Functional impairment of reading in patients with dry eye. British Journal of Ophthalmology 2017;101(4):481‐6. - PubMed
Moss 2000
-
- Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Archives of Ophthalmology 2000;118(9):1264‐8. - PubMed
Moss 2004
-
- Moss SE, Klein R, Klein BE. Incidence of dry eye in an older population. Archives of Ophthalmology 2004;122(3):369‐73. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saldanha 2017
Saldanha 2018
Schaumberg 2003
-
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. American Journal of Ophthalmology 2003;136(2):318‐26. - PubMed
Schaumberg 2009
Stapleton 2017
-
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocular Surface 2017;15(3):334‐65. - PubMed
Strong 2005
-
- Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea 2005;24(1):80‐5. - PubMed
Sun 2017
Uchino 2011
-
- Uchino M, Nishiwaki Y, Michikawa T, Shirakawa K, Kuwahara E, Yamada M, et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology 2011;118(12):2361‐7. - PubMed
Uchiyama 2007
-
- Uchiyama E, Aronowicz JD, Butovich IA, McCulley JP. Increased evaporative rates in laboratory testing conditions simulating airplane cabin relative humidity: an important factor for dry eye syndrome. Eye and Contact Lens 2007;33(4):174‐6. - PubMed
van Landingham 2014
-
- Landingham SW, West SK, Akpek EK, Muñoz B, Ramulu PY. Impact of dry eye on reading in a population‐based sample of the elderly: the Salisbury Eye Evaluation. British Journal of Ophthalmology 2014;98(5):639‐44. - PubMed
Waldmeier 2003
-
- Waldmeier PC, Zimmermann K, Qian T, Tintelnot‐Blomley M, Lemasters JJ. Cyclophilin D as a drug target. Current Medicinal Chemistry 2003;10(16):1485‐506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

