Preclinical efficacy of dual mTORC1/2 inhibitor AZD8055 in renal cell carcinoma harboring a TFE3 gene fusion
- PMID: 31519159
- PMCID: PMC6743205
- DOI: 10.1186/s12885-019-6096-0
Preclinical efficacy of dual mTORC1/2 inhibitor AZD8055 in renal cell carcinoma harboring a TFE3 gene fusion
Abstract
Background: Renal cell carcinomas (RCC) harboring a TFE3 gene fusion (TfRCC) represent an aggressive subset of kidney tumors. Key signaling pathways of TfRCC are unknown and preclinical in vivo data are lacking. We investigated Akt/mTOR pathway activation and the preclinical efficacy of dual mTORC1/2 versus selective mTORC1 inhibition in TfRCC.
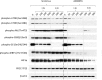
Methods: Levels of phosphorylated Akt/mTOR pathway proteins were compared by immunoblot in TfRCC and clear cell RCC (ccRCC) cell lines. Effects of the mTORC1 inhibitor, sirolimus, and the dual mTORC1/2 inhibitor, AZD8055, on Akt/mTOR activation, cell cycle progression, cell viability and cytotoxicity were compared in TfRCC cells. TfRCC xenograft tumor growth in mice was evaluated after 3-week treatment with oral AZD8055, intraperitoneal sirolimus and respective vehicle controls.
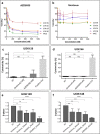
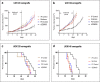
Results: The Akt/mTOR pathway was activated to a similar or greater degree in TfRCC than ccRCC cell lines and persisted partly during growth factor starvation, suggesting constitutive activation. Dual mTORC1/2 inhibition with AZD8055 potently inhibited TfRCC viability (IC50 = 20-50 nM) due at least in part to cell cycle arrest, while benign renal epithelial cells were relatively resistant (IC50 = 400 nM). Maximal viability reduction was greater with AZD8055 than sirolimus (80-90% versus 30-50%), as was the extent of Akt/mTOR pathway inhibition, based on significantly greater suppression of P-Akt (Ser473), P-4EBP1, P-mTOR and HIF1α. In mouse xenograft models, AZD8055 achieved significantly better tumor growth inhibition and prolonged mouse survival compared to sirolimus or vehicle controls.
Conclusions: Akt/mTOR activation is common in TfRCC and a promising therapeutic target. Dual mTORC1/2 inhibition suppresses Akt/mTOR signaling more effectively than selective mTORC1 inhibition and demonstrates in vivo preclinical efficacy against TFE3-fusion renal cell carcinoma.
Keywords: AZD8055; Fusion gene; MITF; TFE3; Translocation renal cell carcinoma; mTOR inhibitor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Cancer Genome Atlas Research N. Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis C, Wheeler DA, Murray BA, Schmidt L, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med. 2016;374(2):135–145. doi: 10.1056/NEJMoa1505917. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

