Global oral cholera vaccine use, 2013-2018
- PMID: 31519444
- PMCID: PMC10967685
- DOI: 10.1016/j.vaccine.2019.08.086
Global oral cholera vaccine use, 2013-2018
Abstract
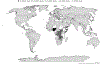
Vaccination is a key intervention to prevent and control cholera in conjunction with water, sanitation and hygiene activities. An oral cholera vaccine (OCV) stockpile was established by the World Health Organization (WHO) in 2013. We reviewed its use from July 2013 to all of 2018 in order to assess its role in cholera control. We computed information related to OCV deployments and campaigns conducted including setting, target population, timelines, delivery strategy, reported adverse events, coverage achieved, and costs. In 2013-2018, a total of 83,509,941 OCV doses have been requested by 24 countries, of which 55,409,160 were approved and 36,066,010 eventually shipped in 83 deployments, resulting in 104 vaccination campaigns in 22 countries. OCVs had in general high uptake (mean administrative coverage 1st dose campaign at 90.3%; 2nd dose campaign at 88.2%; mean survey-estimated two-dose coverage at 69.9%, at least one dose at 84.6%) No serious adverse events were reported. Campaigns were organized quickly (five days median duration). In emergency settings, the longest delay was from the occurrence of the emergency to requesting OCV (median: 26 days). The mean cost of administering one dose of vaccine was 2.98 USD. The OCV stockpile is an important public health resource. OCVs were generally well accepted by the population and their use demonstrated to be safe and feasible in all settings. OCV was an inexpensive intervention, although timing was a limiting factor for emergency use. The dynamic created by the establishment of the OCV stockpile has played a role in the increased use of the vaccine by setting in motion a virtuous cycle by which better monitoring and evaluation leads to better campaign organization, better cholera control, and more requests being generated. Further work is needed to improve timeliness of response and contextualize strategies for OCV delivery in the various settings.
Keywords: Cholera Elimination; End Cholera; Global Task Force on Cholera Control; Oral Cholera Vaccine; Vaccination Campaigns.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

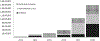
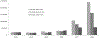
References
-
- Lippi D, Gotuzzo E, Caini S. Cholera. Microbiol Spectr 2016;4:4. - PubMed
-
- World Health Organization. Cholera, 2017. Wkly Epidemiol Rec 2018;93(38):489–500.
-
- Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. Cholera. Lancet 2017. - PubMed
-
- Toole MJ, Waldman RJ. The public health aspects of complex emergencies and refugee situations. Annu Rev Public Health 1997;18:283–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

