The proximal hamstring avulsion clinical trial (PHACT)-a randomised controlled non-inferiority trial of operative versus non-operative treatment of proximal hamstrings avulsions: study protocol
- PMID: 31519683
- PMCID: PMC6747659
- DOI: 10.1136/bmjopen-2019-031607
The proximal hamstring avulsion clinical trial (PHACT)-a randomised controlled non-inferiority trial of operative versus non-operative treatment of proximal hamstrings avulsions: study protocol
Abstract
Introduction: The treatment of proximal hamstring avulsions is controversial. While several trials have investigated the outcome for patients treated surgically, there is today no prospective trial comparing operative treatment with non-operative treatment. This protocol describes the design for the proximal hamstring avulsion clinical trial (PHACT)-the first randomised controlled trial of operative versus non-operative treatment for proximal hamstring avulsions.
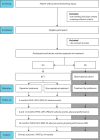
Methods and analysis: PHACT is a multicentre randomised controlled trial conducted across Sweden, Norway and Finland. Eligible patients (60 participants/treatment arm) with a proximal hamstring avulsion of at least two of three tendons will be randomised to either operative or non-operative treatment. Participants allocated to surgery will undergo reinsertion of the tendons with suture anchors. The rehabilitation programme will be the same for both treatment groups. When patient or surgeon equipoise for treatment alternatives cannot be reached and randomisation therefore is not possible, patients will be invited to participate in a parallel observational non-randomised cohort. The primary outcome will be the patient-reported outcome measure Perth hamstring assessment tool at 24 months. Secondary outcomes include the Lower Extremity Functional Score, physical performance and muscle strength tests, patient satisfaction and MR imaging. Data analysis will be blinded and intention-to-treat analysis will be preformed.
Ethics and dissemination: Ethical approval has been granted by the Ethical Committee of Uppsala University (DNR: 2017-170) and by the Norwegian ethical board (REC: 2017/1911). The study will be conducted in agreement with the Helsinki declaration. The findings will be disseminated in peer-reviewed publications.
Trial registration number: NCT03311997.
Keywords: MRI; hamstring; hamstring tendons; muscle strength; patient-reported outcome meassure; randomized controlled trial.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Nothing to declare
Figures
Similar articles
-
Operative versus Nonoperative Treatment of Proximal Hamstring Avulsions.NEJM Evid. 2024 Aug;3(8):EVIDoa2400056. doi: 10.1056/EVIDoa2400056. Epub 2024 Jul 18. NEJM Evid. 2024. PMID: 39023393 Clinical Trial.
-
Functional Results and Outcomes After Repair of Partial Proximal Hamstring Avulsions at Midterm Follow-up.Am J Sports Med. 2019 Dec;47(14):3436-3443. doi: 10.1177/0363546519879117. Epub 2019 Oct 21. Am J Sports Med. 2019. PMID: 31634433
-
Outcomes After Operative and Nonoperative Treatment of Proximal Hamstring Avulsions: A Systematic Review and Meta-analysis.Am J Sports Med. 2018 Sep;46(11):2798-2808. doi: 10.1177/0363546517732526. Epub 2017 Oct 10. Am J Sports Med. 2018. PMID: 29016194
-
Reconstruction of chronic proximal hamstring avulsion injuries using ipsilateral distal hamstring tendons results in good clinical outcomes and patient satisfaction.Knee Surg Sports Traumatol Arthrosc. 2019 Sep;27(9):2958-2966. doi: 10.1007/s00167-018-5310-y. Epub 2018 Nov 23. Knee Surg Sports Traumatol Arthrosc. 2019. PMID: 30470850
-
Proximal avulsion of the hamstring in young athlete patients: a case series and review of literature.Eur J Orthop Surg Traumatol. 2024 Dec;34(8):4139-4147. doi: 10.1007/s00590-024-04096-1. Epub 2024 Oct 16. Eur J Orthop Surg Traumatol. 2024. PMID: 39414664 Free PMC article. Review.
Cited by
-
Increasing incidence of surgically treated hamstring injuries: a nationwide registry study in Sweden between 2001 and 2020.Acta Orthop. 2023 Jul 4;94:336-341. doi: 10.2340/17453674.2023.13650. Acta Orthop. 2023. PMID: 37417907 Free PMC article.
-
Sciatic Nerve Compression after a Chronic Proximal Hamstring Tear: A Report of Two Cases and a Narrative Review of the Literature.Life (Basel). 2023 Aug 17;13(8):1762. doi: 10.3390/life13081762. Life (Basel). 2023. PMID: 37629619 Free PMC article.
-
No difference in clinical outcomes between operative and nonoperative management of minimally retracted proximal hamstring ruptures.Knee Surg Sports Traumatol Arthrosc. 2023 Jul;31(7):2739-2745. doi: 10.1007/s00167-023-07400-4. Epub 2023 Apr 6. Knee Surg Sports Traumatol Arthrosc. 2023. PMID: 37022392
-
Predictive Factors Influencing Functional Results After Proximal Hamstring Tendon Avulsion Surgery: A Patient-Reported Outcome Study After 227 Operations From a Single Center.Orthop J Sports Med. 2021 Oct 29;9(10):23259671211043097. doi: 10.1177/23259671211043097. eCollection 2021 Oct. Orthop J Sports Med. 2021. PMID: 34734098 Free PMC article.
-
Proximal Hamstring Ruptures: Treatment, Rehabilitation, and Return to Play.Curr Rev Musculoskelet Med. 2023 Mar;16(3):103-113. doi: 10.1007/s12178-023-09821-7. Epub 2023 Feb 9. Curr Rev Musculoskelet Med. 2023. PMID: 36757628 Free PMC article. Review.
References
-
- Bodendorfer BM, Curley AJ, Kotler JA, et al. . Outcomes after operative and Nonoperative treatment of proximal hamstring Avulsions: a systematic review and meta-analysis. Am J Sports Med 2017;363546517732526. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical