Human cardiac myosin-binding protein C restricts actin structural dynamics in a cooperative and phosphorylation-sensitive manner
- PMID: 31519753
- PMCID: PMC6827302
- DOI: 10.1074/jbc.RA119.009543
Human cardiac myosin-binding protein C restricts actin structural dynamics in a cooperative and phosphorylation-sensitive manner
Abstract
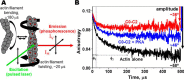
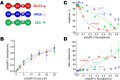
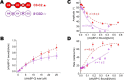
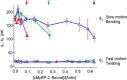
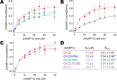
Cardiac myosin-binding protein C (cMyBP-C) is a thick filament-associated protein that influences actin-myosin interactions. cMyBP-C alters myofilament structure and contractile properties in a protein kinase A (PKA) phosphorylation-dependent manner. To determine the effects of cMyBP-C and its phosphorylation on the microsecond rotational dynamics of actin filaments, we attached a phosphorescent probe to F-actin at Cys-374 and performed transient phosphorescence anisotropy (TPA) experiments. Binding of cMyBP-C N-terminal domains (C0-C2) to labeled F-actin reduced rotational flexibility by 20-25°, indicated by increased final anisotropy of the TPA decay. The effects of C0-C2 on actin TPA were highly cooperative (n = ∼8), suggesting that the cMyBP-C N terminus impacts the rotational dynamics of actin spanning seven monomers (i.e. the length of tropomyosin). PKA-mediated phosphorylation of C0-C2 eliminated the cooperative effects on actin flexibility and modestly increased actin rotational rates. Effects of Ser to Asp phosphomimetic substitutions in the M-domain of C0-C2 on actin dynamics only partially recapitulated the phosphorylation effects. C0-C1 (lacking M-domain/C2) similarly exhibited reduced cooperativity, but not as reduced as by phosphorylated C0-C2. These results suggest an important regulatory role of the M-domain in cMyBP-C effects on actin structural dynamics. In contrast, phosphomimetic substitution of the glycogen synthase kinase (GSK3β) site in the Pro/Ala-rich linker of C0-C2 did not significantly affect the TPA results. We conclude that cMyBP-C binding and PKA-mediated phosphorylation can modulate actin dynamics. We propose that these N-terminal cMyBP-C-induced changes in actin dynamics help explain the functional effects of cMyBP-C phosphorylation on actin-myosin interactions.
Keywords: actin; cardiac muscle; cardiac myosin–binding protein C (cMyBP-C); contractile protein; motor protein; myofilament; phosphorylation; protein kinase A (PKA); spectroscopy; structural dynamics.
© 2019 Bunch et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

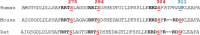

References
-
- Thomas D. D., Muretta J. M., Colson B. A., Mello R. N., and Kast D. (2012) Spectroscopic probes of muscle proteins. in Comprehensive Biophysics (Egelman E. H., ed), Vol. 4, pp. 226–250, Elsevier, Amsterdam: 10.1016/B978-0-12-374920-8.00415-X - DOI
-
- Hopkins S. C., Sabido-David C., van der Heide U. A., Ferguson R. E., Brandmeier B. D., Dale R. E., Kendrick-Jones J., Corrie J. E., Trentham D. R., Irving M., and Goldman Y. E. (2002) Orientation changes of the myosin light chain domain during filament sliding in active and rigor muscle. J. Mol. Biol. 318, 1275–1291 10.1016/S0022-2836(02)00189-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

