MiR-182 regulates cell proliferation and apoptosis in laryngeal squamous cell carcinoma by targeting the CRR9
- PMID: 31519771
- PMCID: PMC6822501
- DOI: 10.1042/BSR20191348
MiR-182 regulates cell proliferation and apoptosis in laryngeal squamous cell carcinoma by targeting the CRR9
Retraction in
-
Retraction: MiR-182 regulates cell proliferation and apoptosis in laryngeal squamous cell carcinoma by targeting the CRR9.Biosci Rep. 2022 Aug 31;42(8):BSR-2019-1348_RET. doi: 10.1042/BSR-2019-1348_RET. Biosci Rep. 2022. PMID: 36043420 Free PMC article. No abstract available.
Abstract
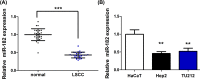
Background: The effect of miR-182 on the expressions of CRR9 in laryngeal squamous cell carcinoma (LSCC) cells, and the impact on invasion and metastasis of LSCC were investigated in the present paper.
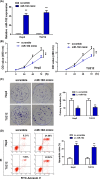
Methods: The expressions of miR-182 in LSCC tissue and cell line were detected by RT-qPCR. MTT assay and Annexin V staining were used to detect the effects of miR-182 on tumor cells proliferation. Target gene prediction and screening, and luciferase reporter assay were designed to verify downstream target genes of miR-182. The mRNA and protein expressions of CRR9 were detected by qRT-PCR and Western blot. Finally, the expressions of CRR9 were measured by transfecting cells with miR-182 in mice.
Results: Compared with normal tissue and cell, the expressions of miR-182 in tumor tissues and cells were much lower. Over-expressions of miR-182 can increase apoptosis rate. Luciferase reporter assay revealed that CRR9 was a downstream gene of miR-182. Reintroduction of CRR9 abolished miR-182-induced LSCC cell growth inhibition. In animal models, over-expressions of miR-182 can reduce tumor weight and promote apoptosis.
Conclusion: miR-182 can inhibit the proliferation of LSCC cells by directly inhibiting the expressions of CRR9, thereby suppressing the occurrences and developments of LSCC.
Keywords: CRR9; apoptosis; laryngeal squamous cell carcinoma; miR-182; proliferation.
© 2019 The Author(s).
Conflict of interest statement
The study agreed with the Ethics Committee of Li huili hospital affiliated to Ningbo University. Experiments using human materials were performed in strict accordance with Declaration of Helsinki. Informed consent was signed for all patients.
The authors declare there are no competing interests associated with the manuscript.
Figures


References
-
- Castro M.A.F., Dedivitis R.A.and Ribeiro K.C.B. (2007) Comorbidity measurement in patients with laryngeal squamous cell carcinoma. Oper. Res. Lett. 69, 146–152 - PubMed
-
- Silveira N.J.F., Varuzza L., Machadolima A., Lauretto M.D.S., Pinheiro D.G., Rodrigues R.V. et al. . (2008) Searching for molecular markers in head and neck squamous cell carcinomas (HNSCC) by statistical and bioinformatic analysis of larynx-derived SAGE libraries. BMC Med. Genet. 1, 56 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

