Regulation of Hippocampal Memory by mTORC1 in Somatostatin Interneurons
- PMID: 31519824
- PMCID: PMC6807283
- DOI: 10.1523/JNEUROSCI.0728-19.2019
Regulation of Hippocampal Memory by mTORC1 in Somatostatin Interneurons
Abstract
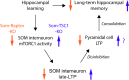
Translational control of long-term synaptic plasticity via Mechanistic Target Of Rapamycin Complex 1 (mTORC1) is crucial for hippocampal learning and memory. The role of mTORC1 is well characterized in excitatory principal cells but remains largely unaddressed in inhibitory interneurons. Here, we used cell-type-specific conditional knock-out strategies to alter mTORC1 function selectively in somatostatin (SOM) inhibitory interneurons (SOM-INs). We found that, in male mice, upregulation and downregulation of SOM-IN mTORC1 activity bidirectionally regulates contextual fear and spatial memory consolidation. Moreover, contextual fear learning induced a metabotropic glutamate receptor type 1 (mGluR1)-mediated late long-term potentiation (LTP) of excitatory input synapses onto hippocampal SOM-INs that was dependent on mTORC1. Finally, the induction protocol for mTORC1-mediated late-LTP in SOM-INs regulated Schaffer collateral pathway LTP in pyramidal neurons. Therefore, mTORC1 activity in somatostatin interneurons contributes to learning-induced persistent plasticity of their excitatory synaptic inputs and hippocampal memory consolidation, uncovering a role of mTORC1 in inhibitory circuits for memory.SIGNIFICANCE STATEMENT Memory consolidation necessitates synthesis of new proteins. Mechanistic Target Of Rapamycin Complex 1 (mTORC1) signaling is crucial for translational control involved in long-term memory and in late long-term potentiation (LTP). This is well described in principal glutamatergic pyramidal cells but poorly understood in GABAergic inhibitory interneurons. Here, we show that mTORC1 activity in somatostatin interneurons, a major subclass of GABAergic cells, is important to modulate long-term memory strength and precision. Furthermore, mTORC1 was necessary for learning-induced persistent LTP at excitatory inputs of somatostatin interneurons that depends on type I metabotropic glutamatergic receptors in the hippocampus. This effect was consistent with a newly described role of these interneurons in the modulation of LTP at Schaffer collateral synapses onto pyramidal cells.
Keywords: hippocampus; mTORC1; memory consolidation; metaplasticity; somatostatin interneurons; synaptic plasticity.
Copyright © 2019 the authors.
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
