Spatial Coupling Tunes NMDA Receptor Responses via Ca2+ Diffusion
- PMID: 31519826
- PMCID: PMC6832682
- DOI: 10.1523/JNEUROSCI.0901-19.2019
Spatial Coupling Tunes NMDA Receptor Responses via Ca2+ Diffusion
Abstract
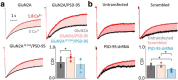
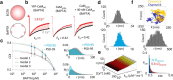
In the CNS, NMDA receptors generate large and highly regulated Ca2+ signals, which are critical for synaptic development and plasticity. They are highly clustered at postsynaptic sites and along dendritic arbors, but whether this spatial arrangement affects their output is unknown. Synaptic NMDA receptor currents are subject to Ca2+-dependent inactivation (CDI), a type of activity-dependent inhibition that requires intracellular Ca2+ and calmodulin (CaM). We asked whether Ca2+ influx through a single NMDA receptor influences the activity of nearby NMDA receptors, as a possible coupling mechanism. Using cell-attached unitary current recordings from GluN1-2a/GluN2A receptors expressed in human HEK293 cells and from NMDA receptors native to hippocampal neurons from male and female rats, we recorded unitary currents from multichannel patches and used a coupled Markov model to determine the extent of signal coupling (κ). In the absence of extracellular Ca2+, we observed no cooperativity (κ < 0.1), whereas in 1.8 mm external Ca2+, both recombinant and native channels showed substantial negative cooperativity (κ = 0.27). Intracellular Ca2+ chelation or overexpression of a Ca2+-insensitive CaM mutant, reduced coupling, which is consistent with CDI representing the coupling mechanism. In contrast, cooperativity increased substantially (κ = 0.68) when overexpressing the postsynaptic scaffolding protein PSD-95, which increased receptor clustering. Together, these new results demonstrate that NMDA receptor currents are negatively coupled through CDI, and the degree of coupling can be tuned by the distance between receptors. Therefore, channel clustering can influence the activity-dependent reduction in NMDA receptor currents.SIGNIFICANCE STATEMENT At central synapses, NMDA receptors are a major class of excitatory glutamate-gated channels and a source of activity-dependent Ca2+ influx. In turn, fluxed Ca2+ ions bind to calmodulin-primed receptors and reduce further entry, through an autoinhibitory mechanism known as Ca2+ -dependent inactivation (CDI). Here, we show that the diffusion of fluxed Ca2+ between active channels situated within submicroscopic distances amplified receptor inactivation. Thus, calmodulin-mediated gating modulation, an evolutionarily conserved regulatory mechanism, endows synapses with sensitivity to both the temporal sequence and spatial distribution of Ca2+ signals. Perturbations in this mechanism, which coordinates the activity of NMDA receptors within a cluster, may cause signaling alterations that contribute to neuropsychiatric conditions.
Keywords: NMDA receptor; PSD-95; calmodulin; clustering; cooperative gating; patch-clamp.
Copyright © 2019 the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
