Dynamic changes of muscle insulin sensitivity after metabolic surgery
- PMID: 31519890
- PMCID: PMC6744497
- DOI: 10.1038/s41467-019-12081-0
Dynamic changes of muscle insulin sensitivity after metabolic surgery
Erratum in
-
Author Correction: Dynamic changes of muscle insulin sensitivity after metabolic surgery.Nat Commun. 2022 Jun 10;13(1):3353. doi: 10.1038/s41467-022-29350-0. Nat Commun. 2022. PMID: 35688803 Free PMC article. No abstract available.
Abstract
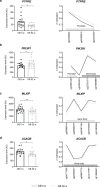
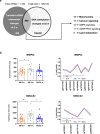
The mechanisms underlying improved insulin sensitivity after surgically-induced weight loss are still unclear. We monitored skeletal muscle metabolism in obese individuals before and over 52 weeks after metabolic surgery. Initial weight loss occurs in parallel with a decrease in muscle oxidative capacity and respiratory control ratio. Persistent elevation of intramyocellular lipid intermediates, likely resulting from unrestrained adipose tissue lipolysis, accompanies the lack of rapid changes in insulin sensitivity. Simultaneously, alterations in skeletal muscle expression of genes involved in calcium/lipid metabolism and mitochondrial function associate with subsequent distinct DNA methylation patterns at 52 weeks after surgery. Thus, initial unfavorable metabolic changes including insulin resistance of adipose tissue and skeletal muscle precede epigenetic modifications of genes involved in muscle energy metabolism and the long-term improvement of insulin sensitivity.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

