The redox metabolic pathways function to limit Anaplasma phagocytophilum infection and multiplication while preserving fitness in tick vector cells
- PMID: 31520000
- PMCID: PMC6744499
- DOI: 10.1038/s41598-019-49766-x
The redox metabolic pathways function to limit Anaplasma phagocytophilum infection and multiplication while preserving fitness in tick vector cells
Abstract
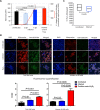
Aerobic organisms evolved conserved mechanisms controlling the generation of reactive oxygen species (ROS) to maintain redox homeostasis signaling and modulate signal transduction, gene expression and cellular functional responses under physiological conditions. The production of ROS by mitochondria is essential in the oxidative stress associated with different pathologies and in response to pathogen infection. Anaplasma phagocytophilum is an intracellular pathogen transmitted by Ixodes scapularis ticks and causing human granulocytic anaplasmosis. Bacteria multiply in vertebrate neutrophils and infect first tick midgut cells and subsequently hemocytes and salivary glands from where transmission occurs. Previous results demonstrated that A. phagocytophilum does not induce the production of ROS as part of its survival strategy in human neutrophils. However, little is known about the role of ROS during pathogen infection in ticks. In this study, the role of tick oxidative stress during A. phagocytophilum infection was characterized through the function of different pathways involved in ROS production. The results showed that tick cells increase mitochondrial ROS production to limit A. phagocytophilum infection, while pathogen inhibits alternative ROS production pathways and apoptosis to preserve cell fitness and facilitate infection. The inhibition of NADPH oxidase-mediated ROS production by pathogen infection appears to occur in both neutrophils and tick cells, thus supporting that A. phagocytophilum uses common mechanisms for infection of ticks and vertebrate hosts. However, differences in ROS response to A. phagocytophilum infection between human and tick cells may reflect host-specific cell tropism that evolved during pathogen life cycle.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

