No effect of triheptanoin on exercise performance in McArdle disease
- PMID: 31520525
- PMCID: PMC6801166
- DOI: 10.1002/acn3.50863
No effect of triheptanoin on exercise performance in McArdle disease
Abstract
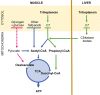
Objective: To study if treatment with triheptanoin, a 7-carbon triglyceride, improves exercise tolerance in patients with McArdle disease. McArdle patients have a complete block in glycogenolysis and glycogen-dependent expansion of tricarboxylic acid cycle (TCA), which may restrict fat oxidation. We hypothesized that triheptanoin metabolism generates substrates for the TCA, which potentially boosts fat oxidation and improves exercise tolerance in McArdle disease.
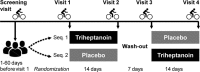
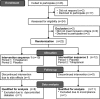
Methods: Double-blind, placebo-controlled, crossover study in patients with McArdle disease completing two treatment periods of 14 days each with a triheptanoin or placebo diet (1 g/kg/day). Primary outcome was change in mean heart rate during 20 min submaximal exercise on a cycle ergometer. Secondary outcomes were change in peak workload and oxygen uptake along with changes in blood metabolites and respiratory quotients.
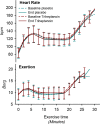
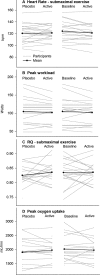
Results: Nineteen of 22 patients completed the trial. Malate levels rose on triheptanoin treatment versus placebo (8.0 ± SD2.3 vs. 5.5 ± SD1.8 µmol/L, P < 0.001), but dropped from rest to exercise (P < 0.001). There was no difference in exercise heart rates between triheptanoin (120 ± SD16 bpm) and placebo (121 ± SD16 bpm) treatments. Compared with placebo, triheptanoin did not change the submaximal respiratory quotient (0.82 ± SD0.05 vs. 0.84 ± SD0.03), peak workload (105 ± SD38 vs. 102 ± SD31 Watts), or peak oxygen uptake (1938 ± SD499 vs. 1977 ± SD380 mL/min).
Interpretation: Despite increased resting plasma malate with triheptanoin, the increase was insufficient to generate a normal TCA turnover during exercise and the treatment has no effect on exercise capacity or oxidative metabolism in patients with McArdle disease.
© 2019 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
KLM, PL, AEB, MGS, CO, SNH, DRP, NSP, MA, MPL, AC, JV and RGH have nothing to report. RQ has been paid honoraria for lecturing and consultancy work, none of which relates to this disease, intervention or study, from Ultragenyx, Genzyme, Santhera, Sarepta and PTC bio. FM has received consulting fees from and conducted other investigator‐sponsored studies supported by Ultragenyx pharmaceuticals, unrelated to this study.
Figures

References
-
- Orngreen MC, Jeppesen TD, Andersen ST, et al. Fat metabolism during exercise in patients with McArdle disease. Neurology 2009;72:718–724. - PubMed
-
- Andersen ST, Jeppesen TD, Taivassalo T, et al. Effect of changes in fat availability on exercise capacity in McArdle disease. Arch Neurol 2009;66:762–766. - PubMed
-
- Sahlin K, Jorfeldt L, Henriksson K‐G, et al. Tricarboxylic acid cycle intermediates during incremental exercise in healthy subjects and in patients with McArdle’s disease. Clin Sci 1995;88:687–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

