Novel anti-angiogenic PEDF-derived small peptides mitigate choroidal neovascularization
- PMID: 31520600
- PMCID: PMC7032632
- DOI: 10.1016/j.exer.2019.107798
Novel anti-angiogenic PEDF-derived small peptides mitigate choroidal neovascularization
Abstract
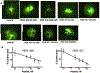
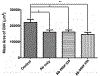
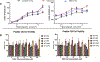
Abnormal migration and proliferation of endothelial cells (EC) drive neovascular retinopathies. While anti-VEGF treatment slows progression, pathology is often supported by decrease in intraocular pigment epithelium-derived factor (PEDF), an endogenous inhibitor of angiogenesis. A surface helical 34-mer peptide of PEDF, comprising this activity, is efficacious in animal models of neovascular retina disease but remains impractically large for therapeutic use. We sought smaller fragments within this sequence that mitigate choroidal neovascularization (CNV). Expecting rapid intravitreal (IVT) clearance, we also developed a method to reversibly attach peptides to nano-carriers for extended delivery. Synthetic fragments of 34-mer yielded smaller anti-angiogenic peptides, and N-terminal capping with dicarboxylic acids did not diminish activity. Charge restoration via substitution of an internal aspartate by asparagine improved potency, achieving low nM apoptotic response in VEGF-activated EC. Two optimized peptides (PEDF 335, 8-mer and PEDF 336, 9-mer) were tested in a mouse model of laser-induced CNV. IVT injection of either peptide, 2-5 days before laser treatment, gave significant CNV decrease at day +14 post laser treatment. The 8-mer also decreased CNV, when administered as eye drops. Also examined was a nanoparticle-conjugate (NPC) prodrug of the 9-mer, having positive zeta potential, expected to display longer intraocular residence. This NPC showed extended efficacy, even when injected 14 days before laser treatment. Neither inflammatory cells nor other histopathologic abnormalities were seen in rabbit eyes harvested 14 days following IVT injection of PEDF 336 (>200 μg). No rabbit or mouse eye irritation was observed over 12-17 days of PEDF 335 eye drops (10 mM). Viability was unaffected in 3 retinal and 2 choroidal cell types by PEDF 335 up to 100 μM, PEDF 336 (100 μM) gave slight growth inhibition only in choroidal EC. A small anti-angiogenic PEDF epitope (G-Y-D-L-Y-R-V) was identified, variants (adipic-Sar-Y-N-L-Y-R-V) mitigate CNV, with clinical potential in treating neovascular retinopathy. Their shared active motif, Y - - - R, is found in laminin (Ln) peptide YIGSR, which binds Ln receptor 67LR, a known high-affinity ligand of PEDF 34-mer.
Keywords: Angiogenesis; Choroidal neovascularization; Laminin receptor; Macular degeneration; PEDF; Peptides; Retinopathy; YIGSR.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures



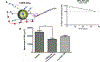
References
-
- Ardini E, Sporchia B, Pollegioni L, Modugno M,Ghirelli C, Castiglioni F,Tagliabue E, Menard S, 2002. Identification of a novel function for 67-kDa laminin receptor: increase in laminin degradation rate and release of motility fragments. Canc Res. 62, 1321–1325. https://www.ncbi.nlm.nih.gov/pubmed/11888899 - PubMed
-
- Askou A,L, Alsing S, Benckendorff JNE, Holmgaard A, Jacob Giehm Mikkelsen JG, Aagaard L, Bek T, Thomas J, Corydon TJ 2019. Suppression of choroidal neovascularization by AAV-based dual-Acting antiangiogenic gene therapy. Mol Ther Nucleic Acids. 16, 38–50. 10.1016/j.omtn.2019.01.012 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

