Preoperative chemotherapy and carbon ions therapy for treatment of resectable and borderline resectable pancreatic adenocarcinoma: a prospective, phase II, multicentre, single-arm study
- PMID: 31521134
- PMCID: PMC6744648
- DOI: 10.1186/s12885-019-6108-0
Preoperative chemotherapy and carbon ions therapy for treatment of resectable and borderline resectable pancreatic adenocarcinoma: a prospective, phase II, multicentre, single-arm study
Abstract
Background: Pancreatic adenocarcinoma is a high-mortality neoplasm with a documented 5-years-overall survival around 5%. In the last decades, a real breakthrough in the treatment of the disease has not been achieved. Here we propose a prospective, phase II, multicentre, single-arm study aiming to assess the efficacy and the feasibility of a therapeutic protocol combining chemotherapy, carbon ion therapy and surgery for resectable and borderline resectable pancreatic adenocarcinoma.
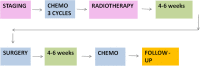
Method: The purpose of this trial (PIOPPO Protocol) is to assess the efficacy and the feasibility of 3 cycles of FOLFIRINOX neoadjuvant chemotherapy followed by a short-course of carbon ion radiotherapy (CIRT) for resectable or borderline resectable pancreatic adenocarcinoma patients. Primary outcome of this study is the assessment of local progression free survival (L-PFS). The calculation of sample size is based on the analysis of the primary endpoint "progression free survival" according to Fleming's Procedure.
Discussion: Very preliminary results provide initial evidence of the feasibility of the combined chemotherapy and CIRT in the neoadjuvant setting for resectable or borderline resectable pancreatic cancer. Completion of the accrual and long term results are awaited to see if this combination of treatment is advisable and will provide the expected benefits.
Trial registration: ClinicalTrials.gov Identifier: NCT03822936 registered on January 2019.
Keywords: Carbon ion radiation therapy; Chemotherapy; Pancreatic adenocarcinoma; Surgery.
Conflict of interest statement
The authors declare that they have no competing interests.
Similar articles
-
Preoperative chemo-CIRT in Re/BRe pancreatic cancer: Insights from a multicenter prospective phase II clinical study (NCT03822936).Tumori. 2024 Dec;110(6):470-474. doi: 10.1177/03008916241291341. Epub 2024 Oct 27. Tumori. 2024. PMID: 39462835 Clinical Trial.
-
Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas.BMC Cancer. 2017 Jul 27;17(1):505. doi: 10.1186/s12885-017-3441-z. BMC Cancer. 2017. PMID: 28750659 Free PMC article.
-
Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial - 1 (NorPACT-1)) - study protocol for a national multicentre randomized controlled trial.BMC Surg. 2017 Aug 25;17(1):94. doi: 10.1186/s12893-017-0291-1. BMC Surg. 2017. PMID: 28841916 Free PMC article. Clinical Trial.
-
Complete pathological response following neoadjuvant FOLFIRINOX in borderline resectable pancreatic cancer - a case report and review.BMC Cancer. 2016 Oct 10;16(1):786. doi: 10.1186/s12885-016-2821-0. BMC Cancer. 2016. PMID: 27724927 Free PMC article. Review.
-
Multimodality management of borderline resectable pancreatic adenocarcinoma.Chin Clin Oncol. 2017 Jun;6(3):27. doi: 10.21037/cco.2017.06.17. Chin Clin Oncol. 2017. PMID: 28705004 Review.
Cited by
-
Factors released by low and high-LET irradiated fibroblasts modulate migration and invasiveness of pancreatic cancer cells.Front Oncol. 2022 Oct 12;12:1003494. doi: 10.3389/fonc.2022.1003494. eCollection 2022. Front Oncol. 2022. PMID: 36313689 Free PMC article.
-
Clinical indications and future directions of carbon-ion radiotherapy: a narrative review.Ewha Med J. 2024 Oct;47(4):e56. doi: 10.12771/emj.2024.e56. Epub 2024 Oct 31. Ewha Med J. 2024. PMID: 40704001 Free PMC article. Review.
-
Synthetic CT in Carbon Ion Radiotherapy of the Abdominal Site.Bioengineering (Basel). 2023 Feb 14;10(2):250. doi: 10.3390/bioengineering10020250. Bioengineering (Basel). 2023. PMID: 36829745 Free PMC article.
-
Carbon ion radiotherapy as definitive treatment in non-metastasized pancreatic cancer: study protocol of the prospective phase II PACK-study.BMC Cancer. 2020 Oct 1;20(1):947. doi: 10.1186/s12885-020-07434-8. BMC Cancer. 2020. PMID: 33004046 Free PMC article. Clinical Trial.
-
Pancreatic cancer: Does a short course of carbon ion radiotherapy worth during COVID-19 outbreak?Pancreatology. 2020 Jul;20(5):1004-1005. doi: 10.1016/j.pan.2020.05.007. Epub 2020 May 12. Pancreatology. 2020. PMID: 32418757 Free PMC article. No abstract available.
References
-
- Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, Pinelli D, Mosconi S, Doglioni C, Chiaravalli M, Pircher C, Arcidiacono PG, Torri V, Maggiora P, Ceraulo D, Falconi M, Gianni L. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):413–423. doi: 10.1016/S2468-1253(18)30081-5. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical