A way to understand idiopathic senescence and apoptosis in primary glioblastoma cells - possible approaches to circumvent these phenomena
- PMID: 31521143
- PMCID: PMC6744717
- DOI: 10.1186/s12885-019-6130-2
A way to understand idiopathic senescence and apoptosis in primary glioblastoma cells - possible approaches to circumvent these phenomena
Abstract
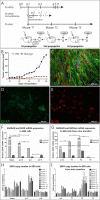
Background: Glioblastoma (GB) is considered one of the most lethal tumors. Extensive research at the molecular level may enable to gain more profound insight into its biology and thus, facilitate development and testing of new therapeutic approaches. Unfortunately, stable glioblastoma cell lines do not reflect highly heterogeneous nature of this tumor, while its primary cultures are difficult to maintain in vitro. We previously reported that senescence is one of the major mechanisms responsible for primary GB cells stabilization failure, to a lesser extent accompanied by apoptosis and mitotic catastrophe-related cell death.
Methods: We made an attempt to circumvent difficulties with glioblastoma primary cultures by testing 3 different approaches aimed to prolong their in vitro maintenance, on a model of 10 patient-derived tumor specimens.
Results: Two out of ten analyzed GB specimens were successfully stabilized, regardless of culture approach applied. Importantly, cells transduced with immortalizing factors or cultured in neural stem cell-like conditions were still undergoing senescence/apoptosis. Sequential in vivo/in vitro cultivation turned out to be the most effective, however, it only enabled to propagate cells with preserved molecular profile up to 3rd mice transfer. Nevertheless, it was the only method that impeded these phenomena long enough to provide sufficient amount of material for in vitro/in vivo targeted analyses. Interestingly, our data additionally demonstrated that some subpopulations of several stabilized GB cell lines undergo idiopathic senescence, however, it is counterbalanced by simultaneous proliferation of other cell subpopulations.
Conclusions: In the majority of primary glioma cultures, there has to be an imbalance towards apoptosis and senescence, following few weeks of rapid proliferation. Our results indicate that it has to be associated with the mechanisms other than maintenance of glioblastoma stem cells or dependence on proteins controlling cell cycle.
Keywords: Apoptosis; Glioblastoma; Immortalization; In vivo model; Senescence.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The failure in the stabilization of glioblastoma-derived cell lines: spontaneous in vitro senescence as the main culprit.PLoS One. 2014 Jan 30;9(1):e87136. doi: 10.1371/journal.pone.0087136. eCollection 2014. PLoS One. 2014. PMID: 24498027 Free PMC article.
-
Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria.Oncogene. 2008 May 1;27(20):2897-909. doi: 10.1038/sj.onc.1210949. Epub 2007 Nov 26. Oncogene. 2008. PMID: 18037961
-
Identification of two glioblastoma-associated stromal cell subtypes with different carcinogenic properties in histologically normal surgical margins.J Neurooncol. 2015 Mar;122(1):1-10. doi: 10.1007/s11060-014-1683-z. Epub 2014 Dec 13. J Neurooncol. 2015. PMID: 25503303
-
An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme-Role in Pathogenesis and Therapeutic Perspective.Int J Mol Sci. 2018 Mar 17;19(3):889. doi: 10.3390/ijms19030889. Int J Mol Sci. 2018. PMID: 29562589 Free PMC article. Review.
-
The Autophagy Status of Cancer Stem Cells in Gliobastoma Multiforme: From Cancer Promotion to Therapeutic Strategies.Int J Mol Sci. 2019 Aug 5;20(15):3824. doi: 10.3390/ijms20153824. Int J Mol Sci. 2019. PMID: 31387280 Free PMC article. Review.
Cited by
-
Increased EGFRvIII Epitope Accessibility after Tyrosine Kinase Inhibitor Treatment of Glioblastoma Cells Creates More Opportunities for Immunotherapy.Int J Mol Sci. 2023 Feb 22;24(5):4350. doi: 10.3390/ijms24054350. Int J Mol Sci. 2023. PMID: 36901782 Free PMC article.
-
Gaps and Doubts in Search to Recognize Glioblastoma Cellular Origin and Tumor Initiating Cells.J Oncol. 2020 Jul 22;2020:6783627. doi: 10.1155/2020/6783627. eCollection 2020. J Oncol. 2020. PMID: 32774372 Free PMC article. Review.
-
The WWOX gene in brain development and pathology.Exp Biol Med (Maywood). 2020 Jul;245(13):1122-1129. doi: 10.1177/1535370220924618. Epub 2020 May 9. Exp Biol Med (Maywood). 2020. PMID: 32389029 Free PMC article. Review.
-
Phenotypical Flexibility of the EGFRvIII-Positive Glioblastoma Cell Line and the Multidirectional Influence of TGFβ and EGF on These Cells-EGFRvIII Appears as a Weak Oncogene.Int J Mol Sci. 2022 Oct 12;23(20):12129. doi: 10.3390/ijms232012129. Int J Mol Sci. 2022. PMID: 36292985 Free PMC article.
-
Role of Senescence in Tumorigenesis and Anticancer Therapy.J Oncol. 2022 Mar 18;2022:5969536. doi: 10.1155/2022/5969536. eCollection 2022. J Oncol. 2022. PMID: 35342397 Free PMC article. Review.
References
-
- Stoczynska-Fidelus E, Piaskowski S, Bienkowski M, Banaszczyk M, Hulas-Bigoszewska K, Winiecka-Klimek M, et al. The failure in the stabilization of glioblastoma-derived cell lines: spontaneous in vitro senescence as the Main culprit. PLoS One. 2014;9(1):e87136. doi: 10.1371/journal.pone.0087136. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

